Question 1:
The following figure shows classifications of carbon compounds.
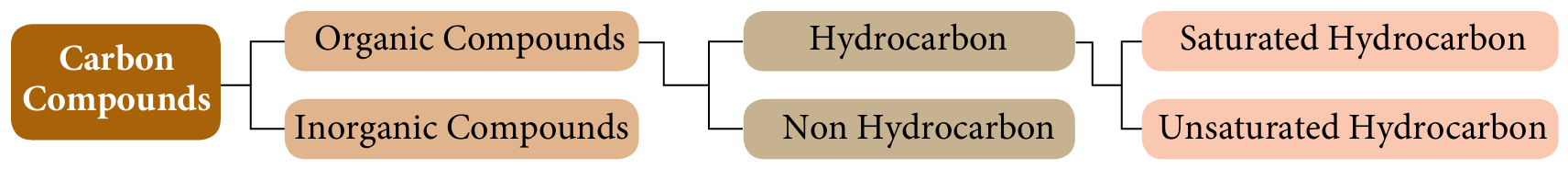
Based on the figure above, state the definitions of:
(a) Organic compounds.
(b) Hydrocarbon and non hydrocarbon.
(c) Saturated and unsaturated hydrocarbons.
Answer:
(a) Carbon compounds are compounds that contain carbon as their constituent element.
(b)
– Organic compounds that contain only hydrogen and carbon.
– Organic compounds that contain carbon and hydrogen and other elements, such as oxygen, nitrogen, phosphorus or halogen.
(c)
– Hydrocarbons that contain only single bonds between carbon atoms.
– Hydrocarbons that contain at least one double or triple bond between carbon atoms.
The following figure shows classifications of carbon compounds.

Based on the figure above, state the definitions of:
(a) Organic compounds.
(b) Hydrocarbon and non hydrocarbon.
(c) Saturated and unsaturated hydrocarbons.
Answer:
(a) Carbon compounds are compounds that contain carbon as their constituent element.
(b)
– Organic compounds that contain only hydrogen and carbon.
– Organic compounds that contain carbon and hydrogen and other elements, such as oxygen, nitrogen, phosphorus or halogen.
(c)
– Hydrocarbons that contain only single bonds between carbon atoms.
– Hydrocarbons that contain at least one double or triple bond between carbon atoms.
Question 2:
(a) What is meant by cracking?
(b) Copy and complete the following reactions:
(i) C10H22 → C6H14 + …………..
(ii) ………….. → C4H8 + C3H6 + C4H12
(c) Discuss the importance of the cracking process.
Answer:
(a) Cracking is the process of breaking long chain hydrocarbons into smaller hydrocarbons at a high temperature with the presence of a catalyst.
(b)(i) C10H22 → C6H14 + C4H8
(b)(ii) C11H26 → C4H8 + C3H6 + C4H12
(c)
– The demand for small sized hydrocarbons is higher.
– The separation of petroleum into its fractions by fractional distillation does not meet the demand for small-sized hydrocarbons.
– The cracking process produces smaller sized hydrocarbons that can be used as fuel as well as raw materials in the petrochemical industry.
(a) What is meant by cracking?
(b) Copy and complete the following reactions:
(i) C10H22 → C6H14 + …………..
(ii) ………….. → C4H8 + C3H6 + C4H12
(c) Discuss the importance of the cracking process.
Answer:
(a) Cracking is the process of breaking long chain hydrocarbons into smaller hydrocarbons at a high temperature with the presence of a catalyst.
(b)(i) C10H22 → C6H14 + C4H8
(b)(ii) C11H26 → C4H8 + C3H6 + C4H12
(c)
– The demand for small sized hydrocarbons is higher.
– The separation of petroleum into its fractions by fractional distillation does not meet the demand for small-sized hydrocarbons.
– The cracking process produces smaller sized hydrocarbons that can be used as fuel as well as raw materials in the petrochemical industry.
