Laboratory Activity 2A ( Fractional Distillation of Petroleum):
Aim: To study the fractional distillation of petroleum
Materials: Petroleum and cotton
Apparatus: Filter paper, retort stand, thermometer (0 °C − 360 °C), round bottom flask, conical flask, test tube, Liebig condenser, wire gauze, tripod stand, test tubes, evaporating dish, porcelain chips, wooden block and Bunsen burner.
Procedure:
1. Measure 50 cm3 of petroleum and pour into a round bottom flask.
2. Add one spatula of porcelain chips into the round bottom flask.
3. Set up the apparatus as shown in Figure 2.4.
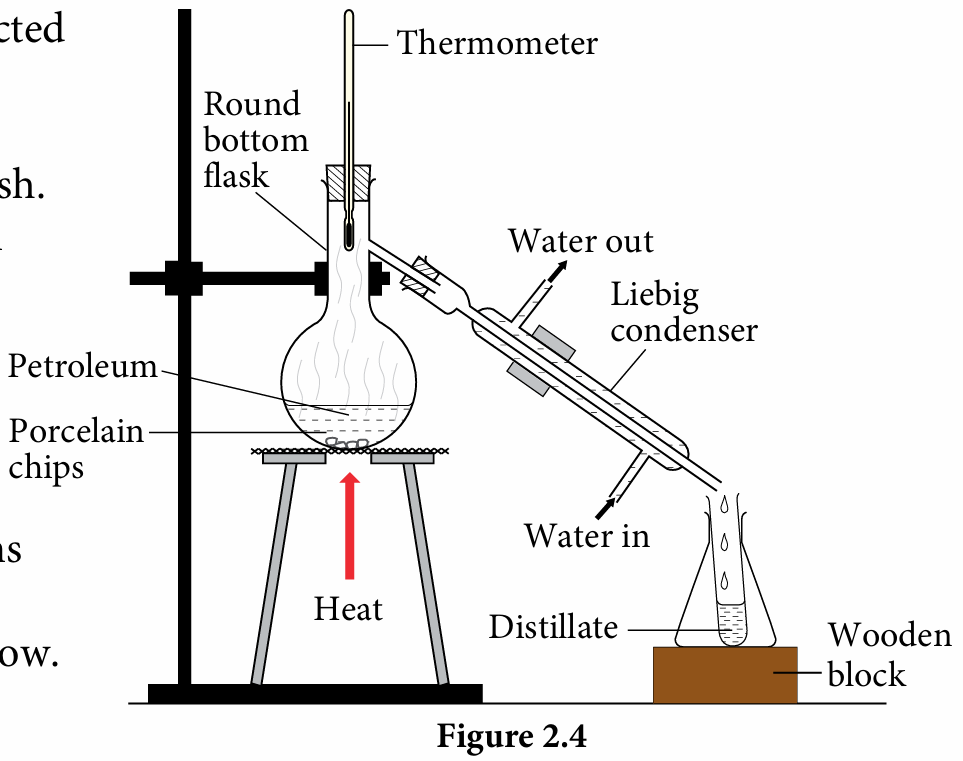
4. Gently heat the petroleum and collect four fractions of petroleum into four separate test tubes at a temperature range of 30 °C − 80 °C, 80 °C − 120 °C, 120 °C − 160 °C and 160 °C − 200 °C.
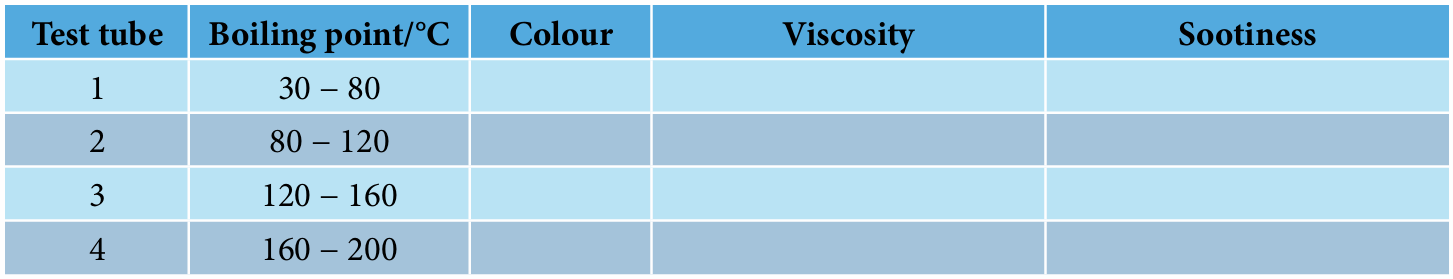
5. Observe each fraction of petroleum collected at different temperatures and record the colour and their viscosity.
6. Place some cotton into an evaporating dish.
7. Put a few drops of the petroleum fraction collected in the test tube onto the cotton in the evaporating dish.
8. Ignite the cotton and observe the colour of the flame and the quantity of soot by placing filter paper above the flame.
9. Repeat steps 6 to 8 for petroleum fractions collected in test tubes 2, 3 and 4.
10. Record your observations in the table below.
Result:
Discussion:
1. Why are porcelain chips added into the round bottom flask?
2. Why is a normal thermometer not used in this activity?
3. State the relation between the boiling point of the fraction of petroleum and the following:
(a) its colour,
(b) its viscosity, and
(c) quantity of soot formed after burning.
4. Which fraction of petroleum is most flammable?
Answer:
1. Some porcelain chips are used for uniform heating of petroleum and to avoid bumping of the liquid due to uneven heating.
2. The boiling points of petroleum fractions are in the range of 30 °C − 200 °C. The maximum temperature that a normal thermometer can record is 110 °C. Thus petroleum fractions that have a boiling point exceeding 110 °C cannot be separated.
3.
(a) The higher the boiling point, the darker the colour of the fraction.
(b) The higher the boiling point, the higher the viscosity of the fraction.
(c) The higher the boiling point, the higher the quantity of soot formed after combustion.
4. Fraction 1
Aim: To study the fractional distillation of petroleum
Materials: Petroleum and cotton
Apparatus: Filter paper, retort stand, thermometer (0 °C − 360 °C), round bottom flask, conical flask, test tube, Liebig condenser, wire gauze, tripod stand, test tubes, evaporating dish, porcelain chips, wooden block and Bunsen burner.
Procedure:
1. Measure 50 cm3 of petroleum and pour into a round bottom flask.
2. Add one spatula of porcelain chips into the round bottom flask.
3. Set up the apparatus as shown in Figure 2.4.

4. Gently heat the petroleum and collect four fractions of petroleum into four separate test tubes at a temperature range of 30 °C − 80 °C, 80 °C − 120 °C, 120 °C − 160 °C and 160 °C − 200 °C.
5. Observe each fraction of petroleum collected at different temperatures and record the colour and their viscosity.
6. Place some cotton into an evaporating dish.
7. Put a few drops of the petroleum fraction collected in the test tube onto the cotton in the evaporating dish.
8. Ignite the cotton and observe the colour of the flame and the quantity of soot by placing filter paper above the flame.
9. Repeat steps 6 to 8 for petroleum fractions collected in test tubes 2, 3 and 4.
10. Record your observations in the table below.
Result:

Discussion:
1. Why are porcelain chips added into the round bottom flask?
2. Why is a normal thermometer not used in this activity?
3. State the relation between the boiling point of the fraction of petroleum and the following:
(a) its colour,
(b) its viscosity, and
(c) quantity of soot formed after burning.
4. Which fraction of petroleum is most flammable?
Answer:
1. Some porcelain chips are used for uniform heating of petroleum and to avoid bumping of the liquid due to uneven heating.
2. The boiling points of petroleum fractions are in the range of 30 °C − 200 °C. The maximum temperature that a normal thermometer can record is 110 °C. Thus petroleum fractions that have a boiling point exceeding 110 °C cannot be separated.
3.
(a) The higher the boiling point, the darker the colour of the fraction.
(b) The higher the boiling point, the higher the viscosity of the fraction.
(c) The higher the boiling point, the higher the quantity of soot formed after combustion.
4. Fraction 1
