Activity 2F:
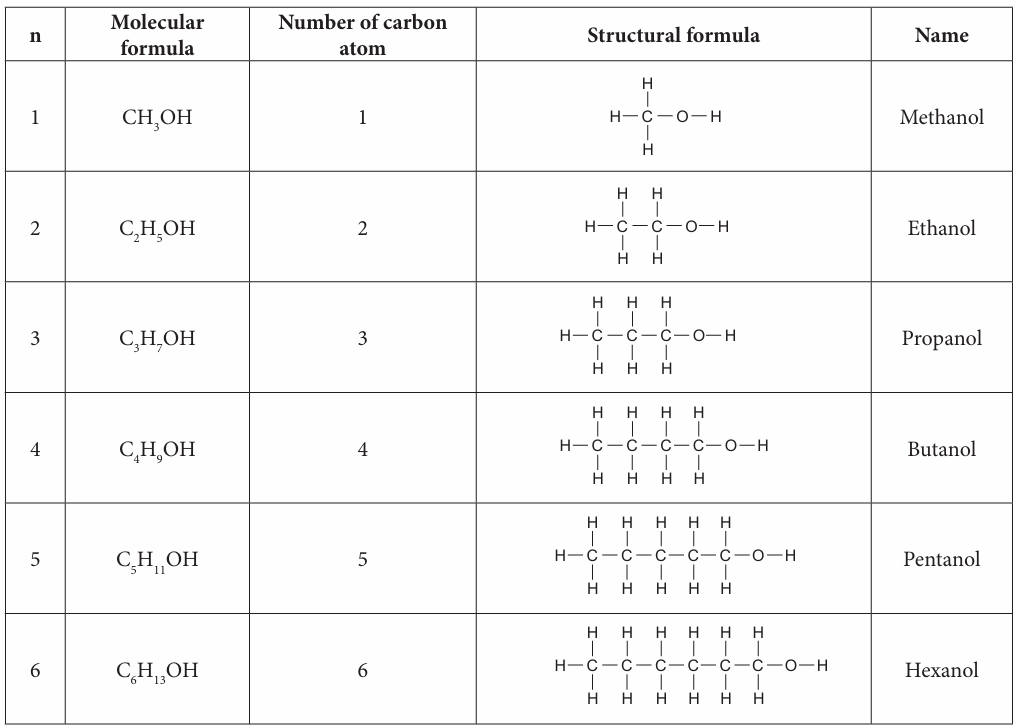
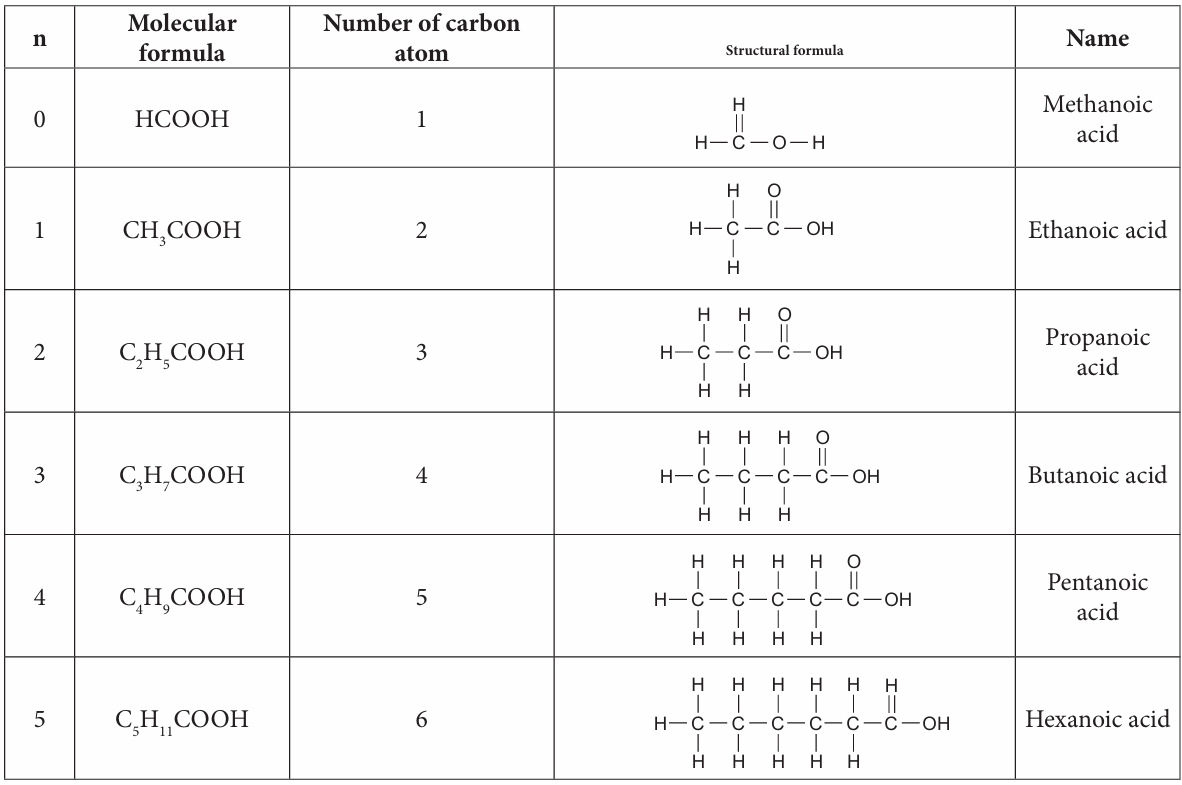
1. Copy and complete Table 2.9 to show the molecular formula, structural formula and the names for the first six straight chain members of alcohol and carboxylic acid.
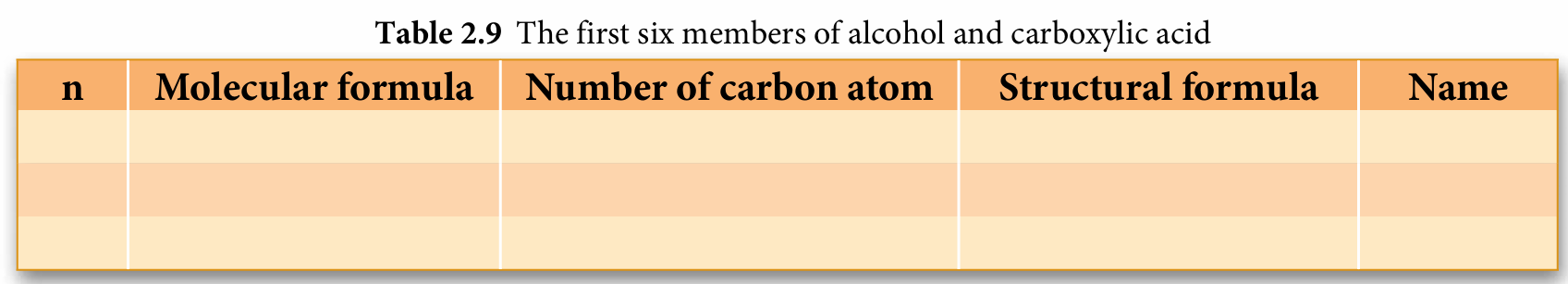
2. Explain why the first value of n is 0 for the general formula of the carboxylic acid homologous series.
3. Explain why the carboxyl functional group COOH always occur at the first carbon.
Answer:
1.
The first six members of alcohols
The first six members carboxylic acids
2. Based on the general formula of carboxylic acid CnH2n+1COOH, there is one carbon atom in the functional group -COOH. As the first member of carboxylic acids having one carbon atom, the value of n in the general formula must start with the value of 0.
3.
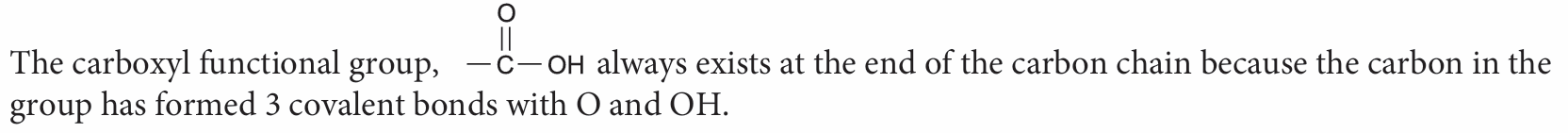
1. Copy and complete Table 2.9 to show the molecular formula, structural formula and the names for the first six straight chain members of alcohol and carboxylic acid.
2. Explain why the first value of n is 0 for the general formula of the carboxylic acid homologous series.
3. Explain why the carboxyl functional group COOH always occur at the first carbon.
Answer:
1.
The first six members of alcohols

The first six members carboxylic acids

2. Based on the general formula of carboxylic acid CnH2n+1COOH, there is one carbon atom in the functional group -COOH. As the first member of carboxylic acids having one carbon atom, the value of n in the general formula must start with the value of 0.
3.