Chemical Properties of Alcohol
Combustion of Alcohol
- As the hydrocarbon, alcohols undergo combustion with the presence of oxygen to produce carbon dioxide and water.
- Combustion of alcohol produces less soot compares to combustion of alkanes and alkenes due to the presence of oxygen in the –OH group.
- Owing to the presence of the –OH group, the percentage of carbon in alcohol is relatively low when compared with the percentages of carbon of alkanes and alkenes.
Equation:
Combustion of Ethanol
C2H5OH + 3O2 → 2CO2 + 3H2O
Combustion of Propanol
C3H7OH + 9/2 O2 → 3CO2 + 4H2O
Reaction of Alcohols with Sodium
Revision:
Reaction of Water with Sodium
2Na + 2H2O → 2NaOH + H2
- We have learned the reaction of group 1 metals with cold water in Form 4 chapter 4, Periodic Table.
- The reaction of an alcohol with sodium is similar to this reaction.
- If a small piece of sodium is dropped into some ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colourless solution of sodium ethoxide, CH3CH2ONa.
Sodium ethoxide is known as an alkoxide. - In the reaction, sodium reacts with the -OH group of alcohol produces sodium alkoxide and hydrogen gas.
Example:
Methanol and Sodium
2CH3OH + 2Na → 2CH3ONa + H2
Ethanol and Sodium
2C2H5OH + 2Na → 2C2H5ONa + H2
Dehydration of Alcohol
- When alcohol is heated, it will decompose to form alkene and water.
- For example, heating ethanol will produce ethene, heating propanol will produce propene, and so on.
- This process is called dehydration of alcohol.
Dehydration of Ethanol
C2H5OH → C2H4 + H2O
Dehydration of Propanol
C3H7OH → C3H6 + H2O
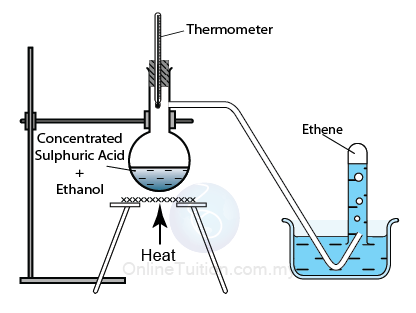
Dehydration of alcohols using an acid catalyst
Catalyst:
Sulphuric acid or Phosphoric acid
Temperature: 170°C
Oxidation of Alcohol
Alcohols can be oxidised to produce carboxylic acid when reacting with oxidising agents.
Equation:
C2H5OH + 2[O] → CH3COOH + H2O
Notes:
- The oxidising agent used:
- Acidified potassium dichromate(VI)
- Acidified potassium manganate (VII)
- Observation:
- For acidified potassium dichromate(VI), if oxidation occurs, the orange solution containing the dichromate(VI) ions are reduced to a green solution containing chromium(III) ions.
- For acidified potassium manganate (VII), the purple colour of potassium manganate (VII) decolourised.
- The alcohol is heated under reflux with an excess oxidising agent.
- The reflux technique used to prevent the alcohol vapour escape to the surrounding.
- When the reaction is complete, the carboxylic acid is distilled off.