Natural Rubber
Natural Rubber
- Natural rubber is a natural polymer.
- Latex is a milk like liquid that flows out after the bark of the rubber tree is cut. Latex is mixture of rubber particles and water.
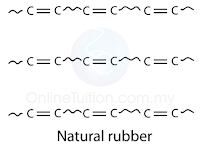
- Formula of natural rubber is (C5H8)n, where n is about 10,000.
- Monomer of natural rubber is C5H8, known as 2-metylbut-1,3-diene (isoprene).
- Each monomer molecule has 2 double bond and thus known as -diene.
Coagulation of Latex
- Each rubber particle consists of rubber molecules which are enveloped by protein membrane.
- Protein membranes are negatively charged on its outer surface.
- Like charges repel. The repelling force exists between the rubber particles which cause the particles to be separated from one another.
- This prevents the bonding of rubber particles and thus prevents coagulation of latex.
- However, latex can coagulates if:
- acid such as ethanoic acid is added to it.
When acid is added to latex, the hydrogen ions from the acid neutralise the negative charges on the surface of the protein membrane. The neutral particles no longer repel each other. These rubber particles may collide with each other, causing the membrane to break. The rubber polymers are freed and they coagulate by combining together to form large lump of rubber polymer. The latex has coagulated. - the latex is left aside for 1 – 2 days. This is due to bacterial action on latex. The activity of bacteria in the latex produces lactic that contains hydrogen ions which causes coagulation of latex.
- Coagulation of latex can be prevented by adding alkali to it. The OH– ions from the alkali will neutralise any acids that may be produced by the bacteria.
Physical Properties of Natural Rubber
Vulcanisation of Rubber
- Natural rubber is vulcanized to improve its characteristics so that its usage could be wider.
- Vulcanised rubber is rubber that has been heated with sulphur.
- Vulcanised rubber is prepared in the laboratory by soaking the rubber in a solution of disulphur dichloride or sulphur monochloride in methylbenzene.
- Rubber molecules have double covalent bonds.
- Sulphur atoms react with the double bond in the rubber molecules chain to form C-S-S-C cross link between the rubber molecules.
Natural Rubber vs Vulcanised Rubber
Natural Rubber | Vulcanised Rubber |
| Soft | Hard |
| Less elastic | More elastic |
| Non-heat resistance | More heat resistance |
| Low melting point | High melting point |
| Easily oxidised | Resist oxidation |