- A molecular formula is a chemical formula which shows the actual number of atoms of each element present in one molecule of a substance
- A structural formula is a chemical formula which shows the arrangement of the atoms in a molecule of a substance.
Writing the Structural Formulae
Rules to be followed:
- All atoms are bonded together by covalent bond represented by a straight line. The bond can be a single bond, a double bond or a triple bond.
- The number of covalent bonds at each atom is as follow:
- Hydrogen: 1
- Carbon: 4
- Oxygen: 2
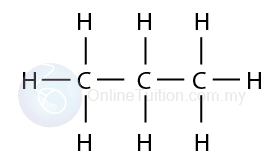
Steps to be followed:
Step 1: Determine the number of carbon in the molecule (Let’s say 3 carbon atom)
Step 2: Draw all the carbon atoms in a straight line and then draw the covalent bonds to connect all the carbon atoms.
Step 3: Draw additional single covalent bonds on each
Step 4: Write the hydrogen atoms
Step 5: Check that each carbon atom has four single covalent bonds, each hydrogen atom has one single covalent bond.