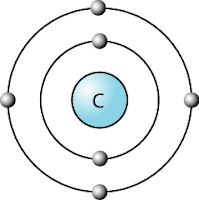
- Carbon is located in group 14. A carbon atom contains 6 electrons, therefore the electronic configuration is 2.4.
- It has 4 valance electrons in the outermost orbital, as shown in the diagram.
- In order to achieve a stable outer octet of electrons, it forms four covalent bonds.
Example
Methane
When a carbon atom combines with four hydrogen atoms, it forms a molecule of methane, CH4
Ethane
If two carbon atoms join, each can still combine with three hydrogen atoms to form a molecule of ethane, C2H6.
Number of Bonds
Each carbon atom is tied to other atoms through four covalent bonds. All the four covalent bonds may exist in three forms as shown below:
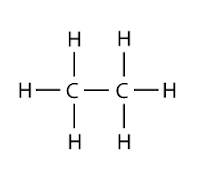 |
| Possesses just a single bond |
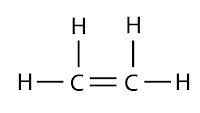 |
| Possesses a double bond |
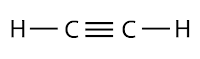 |
| Possesses a triple bond |
Carbon Compounds
Carbon compounds can be divided to:
- Organic carbon compounds
- Inorganic carbon compounds