Physical Properties of Alkanes
- Alkanes are covalent compounds, hence their physical properties are similar to other covalent compounds.
- The atoms in an alkane molecule are bonded together by strong covalent bonds (intramolecular forces).
- The molecules are held together by weak Van der Waals forces (intermolecular forces).
Boiling Points
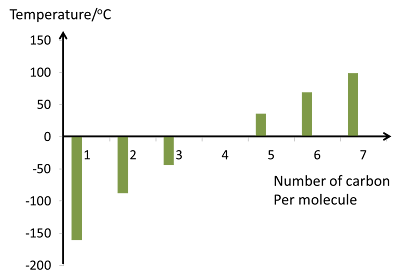 |
| Boiling Point of Alkane |
- The boiling point of alkane increase when the number of carbons in the molecule increases.
- This is due to the increase of Van der Waal force when the size of the molecule increases.
- The boiling points shown are all for the “straight chain” isomers when there are more than one.
- The first four alkanes are gas at room temperature.
Solubility
- Alkanes are insoluble in water, but dissolve in organic solvents.
- The liquid alkanes are good solvents for many other covalent compounds.
Density
- The density of water is higher than density of most alkanes.
- When going down the series, relative molecular mass of alkanes is higher due to the higher force of attraction between molecules and alkane molecules are packed closer together.
Electrical Conductivity
- All members in alkanes do not conduct electricity.
- Alkanes are covalent compounds and do not contain freely moving ions.
