Exothermic Reaction
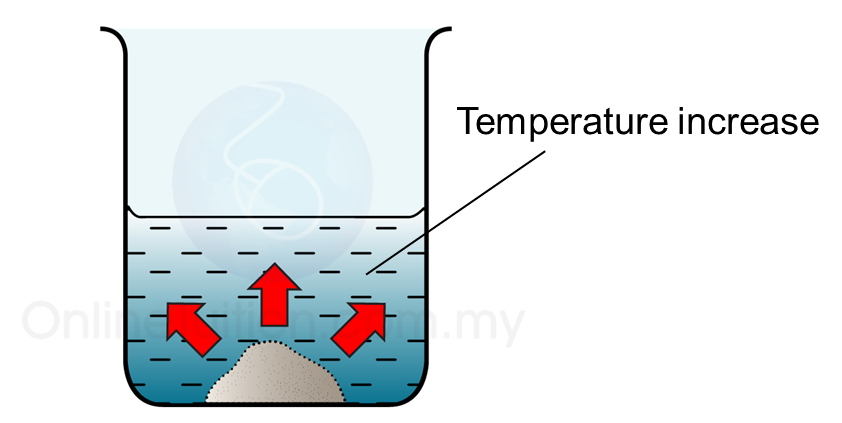
- Exothermic reaction is the chemical reaction that releases heat to the surrounding.
- When energy is given off during a chemical reaction, the temperature of the surrounding will increase.
Example of Exothermic Reaction
| Reaction | Equation |
| Neutralization | Diluted sulphuric acid is neutralized by sodium hydroxide aqueous H2SO4 + 2NaOH → Na2SO4 + 2H2O |
| Acid + carbonate | Reaction between hydrochloric acid and sodium carbonate 2HCl + Na2CO3 → 2NaCl + CO2 + H2O |
| Calcium oxide + water | CaO + H2O → Ca(OH)2 |
| Water + dehydrated copper(II) sulphate | CuSO4 + xH2O → CuSO4•xH2O |
| Dissolve NaOH and KOH in water | NaOH + water → Na+ + OH– KOH + water → K+ + OH– |
| Reaction between alkali metal (Li/Na/K) and water | Sodium + Water 2Na + 2H2O → 2NaOH + H2 |
| Reaction between reactive metal (Mg/Al/Zn/Fe) and diluted acid | Magnesium + Nitric acid Mg + 2HNO3 → Mg(NO3)2 + H2 |
| Combustion | Combustion of methane CH4 + 2O2 → CO2 + 2H2O |
Others
| |