Preparing Alkene
Alkene can be prepared by
- dehydration of alcohol
- craking of alkane
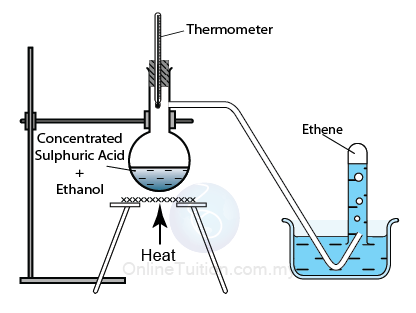
Dehydration of Alcohol
When alcohol is heated, it will decompose to form alkene and water. For example, heating ethanol will produce ethene, heating propanol will produce propene, and so on. This process is called dehydration of alcohol.
Dehydration of Ethanol
C2H5OH → C2H4 + H2O
Dehydration of Propanol
C3H7OH → C3H6 + H2O
Dehydration of alcohols using an acid catalyst
Catalyst:
Sulphuric acid or Phosphoric acid
Temperature: 170°C
Cracking of Alkane
- Cracking is the name given to breaking up large hydrocarbon molecules into smaller and more useful bits.
- This is achieved by using high pressures and temperatures without a catalyst, or lower temperatures and pressures in the presence of a catalyst.
Example: Cracking of butane
Cracking of butane produces a mixture of methane, ethane, ethene and propene.