Activity 7.4:
Discussing the solution involving the rate of reactions and determine the variables involved in the reactions
As a group, solve the following problems:
1. Figure 7.16 shows the graph of volume of carbon dioxide against time for the two experiments, I and II.
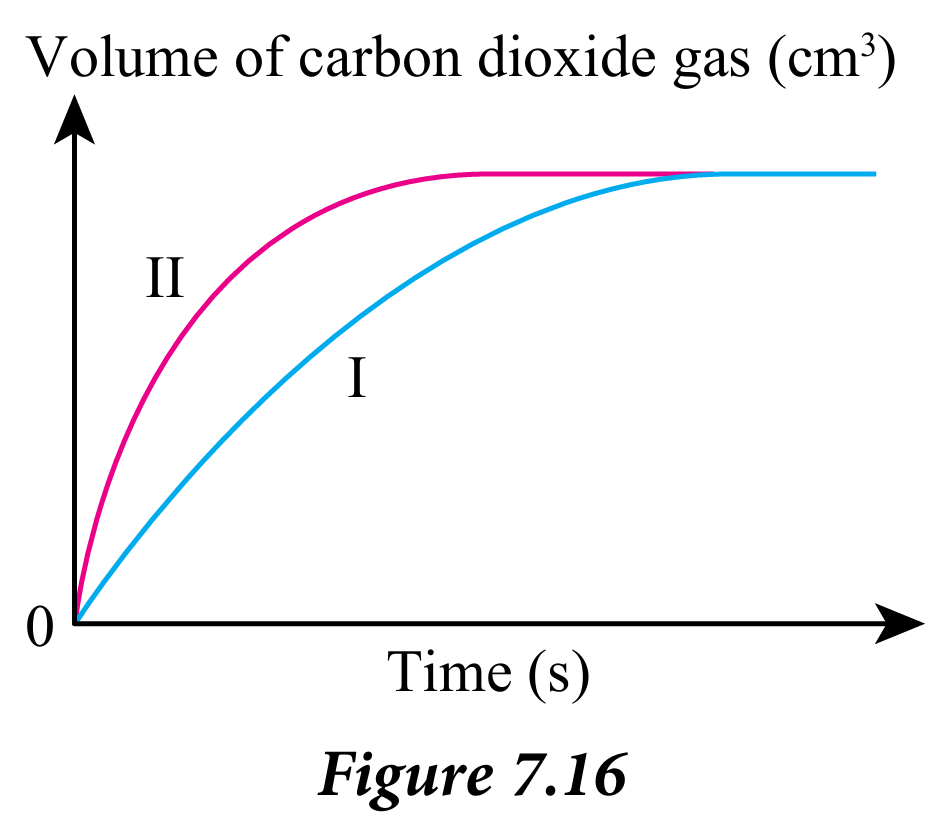
Table 7.7 shows the conditions of the two experiments
Suggest two ways to carry out experiment II so that a similar graph as in Figure 7.16 can be obtained. State all the variables involved.
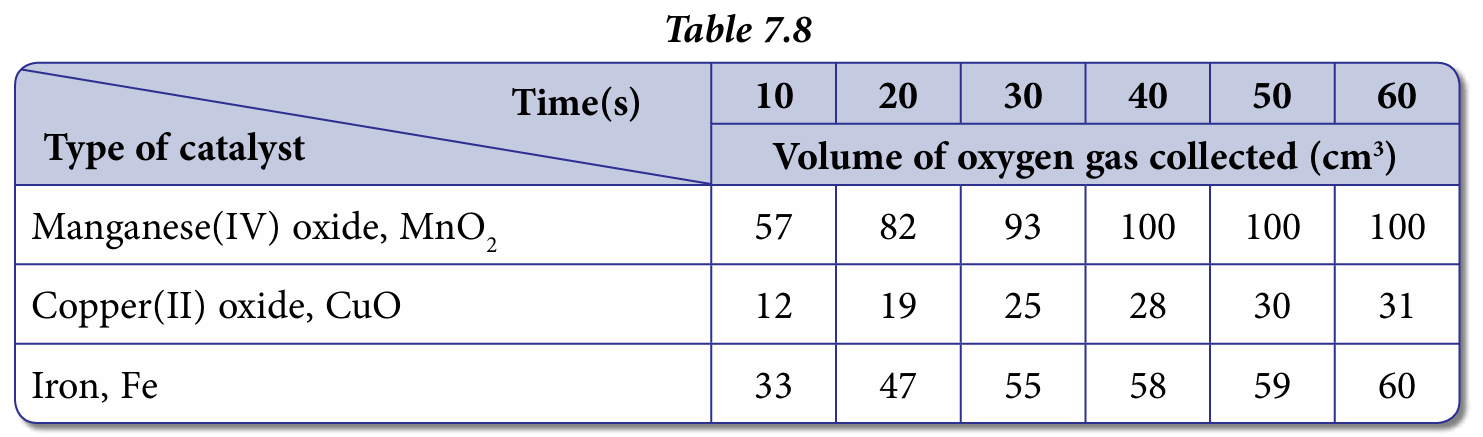
2. Hydrogen peroxide, H2O2 decomposes slowly at room temperature to produce water and oxygen. The decomposition of hydrogen peroxide, H2O2 can be accelerated by the presence of a catalyst. Three experiments are carried out to determine the effect of three different catalysts on the complete decomposition of 50 cm3 of 10-volume hydrogen peroxide solution, H2O2.
Table 7.8 shows the results of the experiments.
(a) Write the chemical equation for the decomposition of hydrogen peroxide, H2O2.
(b) Explain briefly how the experiment was conducted. Include the following in your explanation:
(i) Problem statement
(ii) Hypothesis
(iii) All the variables
(iv) Diagram for the set-up of the apparatus
(c) Which catalyst is more effective in speeding up the rate of decomposition of hydrogen peroxide, H2O2? Explain your answer.
Discussing the solution involving the rate of reactions and determine the variables involved in the reactions
As a group, solve the following problems:
1. Figure 7.16 shows the graph of volume of carbon dioxide against time for the two experiments, I and II.

Table 7.7 shows the conditions of the two experiments

Suggest two ways to carry out experiment II so that a similar graph as in Figure 7.16 can be obtained. State all the variables involved.
2. Hydrogen peroxide, H2O2 decomposes slowly at room temperature to produce water and oxygen. The decomposition of hydrogen peroxide, H2O2 can be accelerated by the presence of a catalyst. Three experiments are carried out to determine the effect of three different catalysts on the complete decomposition of 50 cm3 of 10-volume hydrogen peroxide solution, H2O2.
Table 7.8 shows the results of the experiments.
(a) Write the chemical equation for the decomposition of hydrogen peroxide, H2O2.
(b) Explain briefly how the experiment was conducted. Include the following in your explanation:
(i) Problem statement
(ii) Hypothesis
(iii) All the variables
(iv) Diagram for the set-up of the apparatus
(c) Which catalyst is more effective in speeding up the rate of decomposition of hydrogen peroxide, H2O2? Explain your answer.
Answer:
1.
First method:
The experiment is carried out using 1.0 g marble powder, CaCO3 and 50 cm3 of 0.5 mol dm–3 hydrochloric acid, HCl at room temperature.
Manipulated variable : Size of marble chips, CaCO3
Responding variable : Rate of reaction
Fixed variable : Mass of marble chips, CaCO3, volume and concentration of hydrochloric acid, HCl
Second method:
The experiment is carried out using 1.0 g marble powder, CaCO3 and 50 cm3 of 0.5 mol dm–3 hydrochloric acid, HCl at 50 °C.
Manipulated variable : Temperature of reaction
Responding variable : Rate of reaction
Fixed variable : Mass of marble chips, CaCO3, volume and concentration of hydrochloric acid, HCl
1.
First method:
The experiment is carried out using 1.0 g marble powder, CaCO3 and 50 cm3 of 0.5 mol dm–3 hydrochloric acid, HCl at room temperature.
Manipulated variable : Size of marble chips, CaCO3
Responding variable : Rate of reaction
Fixed variable : Mass of marble chips, CaCO3, volume and concentration of hydrochloric acid, HCl
Second method:
The experiment is carried out using 1.0 g marble powder, CaCO3 and 50 cm3 of 0.5 mol dm–3 hydrochloric acid, HCl at 50 °C.
Manipulated variable : Temperature of reaction
Responding variable : Rate of reaction
Fixed variable : Mass of marble chips, CaCO3, volume and concentration of hydrochloric acid, HCl
Answer:
2.(a) 2H2O2(aq) → 2H2O(l) + O2(g)
(b)
Problem statement: What substance is the most effective catalyst for the decomposition of hydrogen peroxide, H2O2?
Hypothesis: The most effective catalyst will decompose hydrogen peroxide, H2O2 in the shortest time
Variables:
Manipulated variable : Type of catalyst
Responding variable : Rate of reaction
Fixed variable : Mass of catalyst, volume and concentration of hydrogen peroxide, H2O2 and temperature
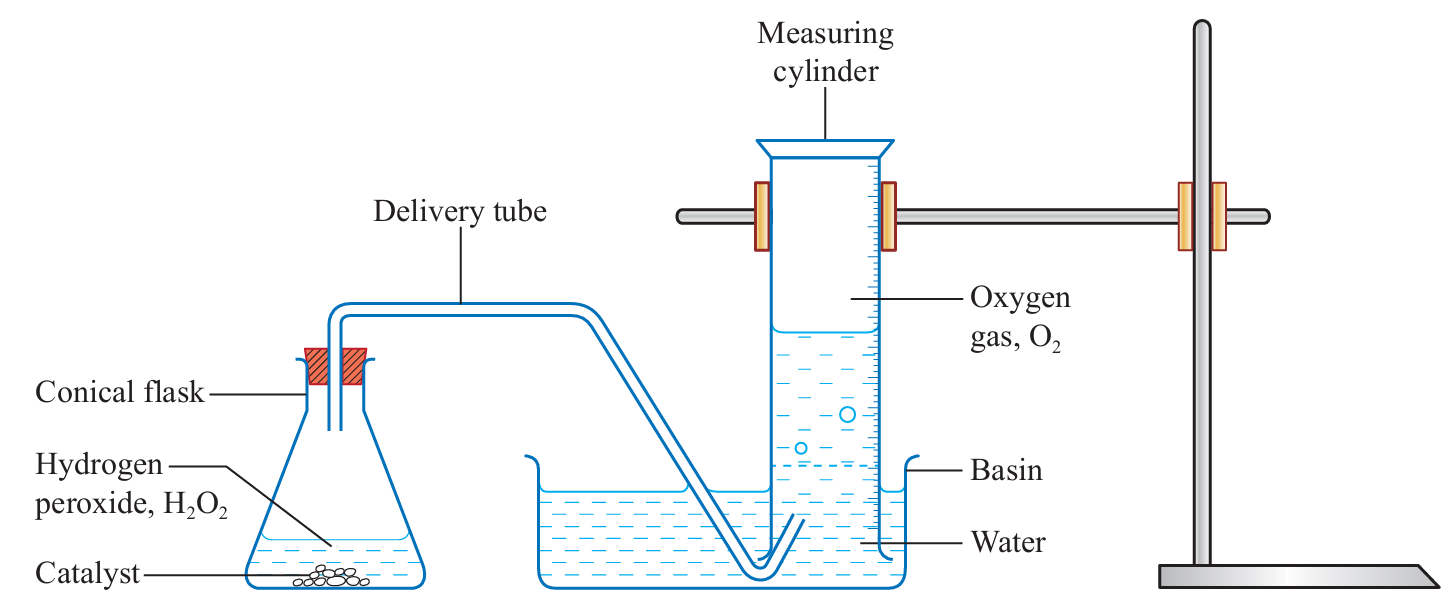
Procedure:
1. Set up the apparatus as shown in the diagram.
2. Put 0.1 g manganese(IV) oxide powder, MnO2 into 50 cm3 10-volume hydrogen peroxide solution, H2O2.
3. Start the stop watch immediately.
4. Record the volume of gas collected in the measuring cylinder every 10 seconds.
5. Repeat steps 1 to 4 using copper(II) oxide, CuO and iron, Fe.
(c)
Manganese(IV) oxide powder, MnO2 because the time taken for the reaction to complete is the shortest, that is 40 seconds.
2.(a) 2H2O2(aq) → 2H2O(l) + O2(g)
(b)
Problem statement: What substance is the most effective catalyst for the decomposition of hydrogen peroxide, H2O2?
Hypothesis: The most effective catalyst will decompose hydrogen peroxide, H2O2 in the shortest time
Variables:
Manipulated variable : Type of catalyst
Responding variable : Rate of reaction
Fixed variable : Mass of catalyst, volume and concentration of hydrogen peroxide, H2O2 and temperature

Procedure:
1. Set up the apparatus as shown in the diagram.
2. Put 0.1 g manganese(IV) oxide powder, MnO2 into 50 cm3 10-volume hydrogen peroxide solution, H2O2.
3. Start the stop watch immediately.
4. Record the volume of gas collected in the measuring cylinder every 10 seconds.
5. Repeat steps 1 to 4 using copper(II) oxide, CuO and iron, Fe.
(c)
Manganese(IV) oxide powder, MnO2 because the time taken for the reaction to complete is the shortest, that is 40 seconds.
