Activity 7.1:
Aim: Determining the time for reaction with reference to some observable and measurable changes.
Materials: Zinc powder, Zn, 0.1 mol dm–3 sulphuric acid, H2SO4, marble chips, CaCO3, 2.0 mol dm–3 nitric acid, HNO3, potassium iodide powder, KI, lead(II) nitrate powder, Pb(NO3)2 and distilled water
Apparatus: Retort stand with clamp, burette, basin, 250 cm3 conical flask, 10 cm3 and 100 cm3 measuring cylinders, rubber stopper, delivery tube, electronic balance, stopwatch, cotton wool, petri dish, weighing bottle, filter funnel, ruler and filter paper
(A) Reaction between zinc, Zn and sulphuric acid, H2SO4
Procedure:
1. Add 20 cm3 of 0.1 mol dm–3 sulphuric acid, H2SO4 into a conical flask.
2. Fill a burette with water and invert it into a basin of water. Clamp the burette vertically.
3. Adjust the water level in the burette so that the level of water is at the 50 cm3 mark.
4. Arrange the apparatus as in Figure 7.2
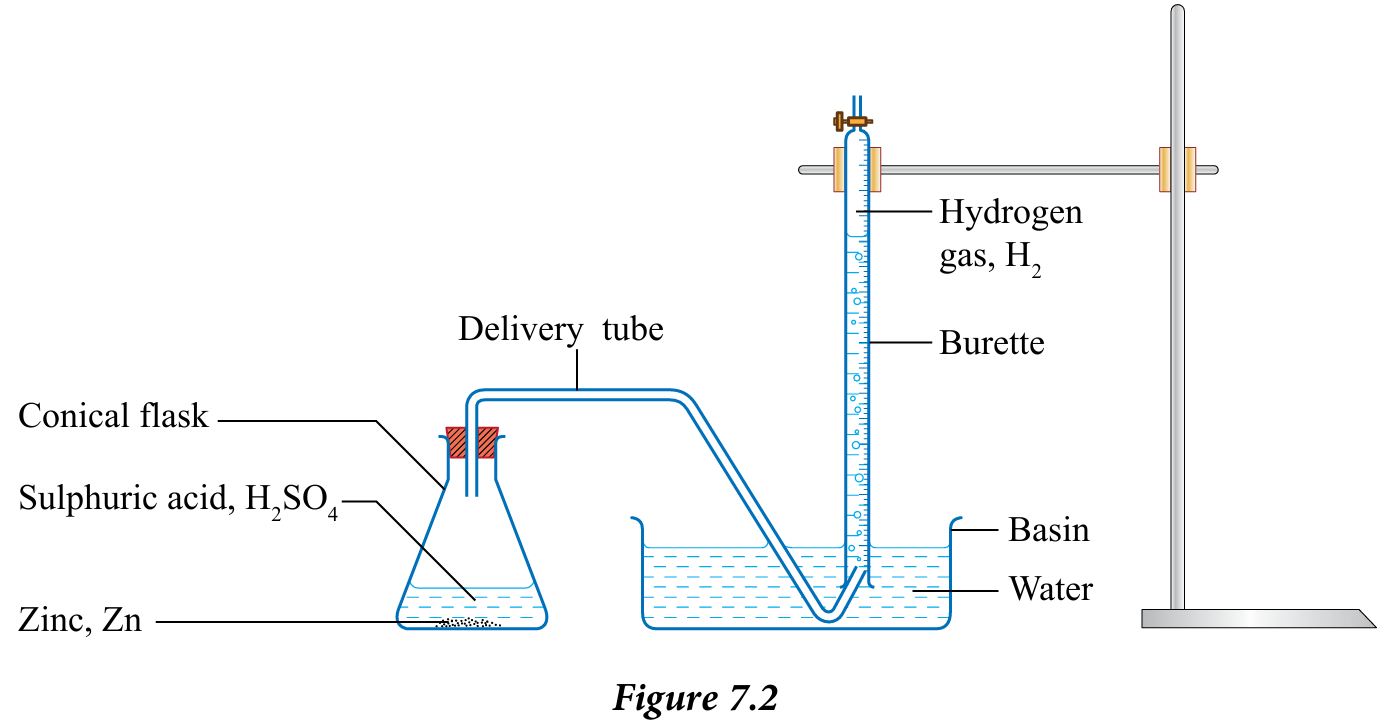
5. Add 5 g of zinc powder, Zn into the conical flask that contains sulphuric acid, H2SO4.
6. Immediately close the conical flask with the rubber stopper that is connected to the delivery tube. Start the stopwatch.
7. Record the burette reading at 0.5 minute intervals for 5 minutes.
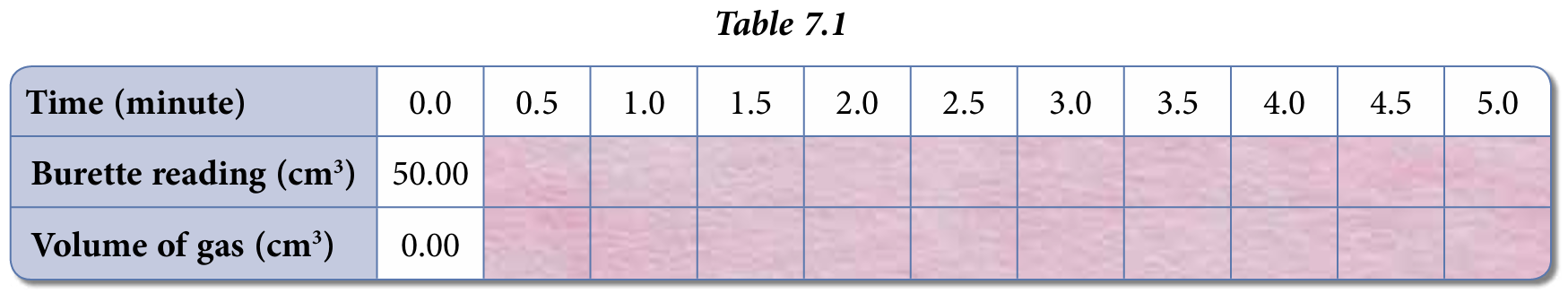
8. Record your results in Table 7.1 given below.
Results:
Discussion:
1. State the observable changes and the measurement made in the activity.
2. Name the gas released.
3. Write a chemical equation for the reaction that occurred.
4. How would you know that the reaction is completed?
Answer:
Discussion:
1. Effervescence/ Gas bubbles are formed. The volume of the gas formed can be measured.
2. Hydrogen gas.
3. Zn(s) + H2SO4 aq) → ZnSO4(aq) + H2(g)
4. Effervescence stopped. No gas bubbles are formed.
Aim: Determining the time for reaction with reference to some observable and measurable changes.
Materials: Zinc powder, Zn, 0.1 mol dm–3 sulphuric acid, H2SO4, marble chips, CaCO3, 2.0 mol dm–3 nitric acid, HNO3, potassium iodide powder, KI, lead(II) nitrate powder, Pb(NO3)2 and distilled water
Apparatus: Retort stand with clamp, burette, basin, 250 cm3 conical flask, 10 cm3 and 100 cm3 measuring cylinders, rubber stopper, delivery tube, electronic balance, stopwatch, cotton wool, petri dish, weighing bottle, filter funnel, ruler and filter paper
(A) Reaction between zinc, Zn and sulphuric acid, H2SO4
Procedure:
1. Add 20 cm3 of 0.1 mol dm–3 sulphuric acid, H2SO4 into a conical flask.
2. Fill a burette with water and invert it into a basin of water. Clamp the burette vertically.
3. Adjust the water level in the burette so that the level of water is at the 50 cm3 mark.
4. Arrange the apparatus as in Figure 7.2

5. Add 5 g of zinc powder, Zn into the conical flask that contains sulphuric acid, H2SO4.
6. Immediately close the conical flask with the rubber stopper that is connected to the delivery tube. Start the stopwatch.
7. Record the burette reading at 0.5 minute intervals for 5 minutes.
8. Record your results in Table 7.1 given below.
Results:

Discussion:
1. State the observable changes and the measurement made in the activity.
2. Name the gas released.
3. Write a chemical equation for the reaction that occurred.
4. How would you know that the reaction is completed?
Answer:
Discussion:
1. Effervescence/ Gas bubbles are formed. The volume of the gas formed can be measured.
2. Hydrogen gas.
3. Zn(s) + H2SO4 aq) → ZnSO4(aq) + H2(g)
4. Effervescence stopped. No gas bubbles are formed.
(B) Reaction between nitric acid, HNO3 and marble chips, CaCO3:
Procedure:
1. Put 100 cm3 of 2.0 mol dm–3 nitric acid, HNO3 into a conical flask.
2. Close the mouth of the conical flask loosely with cotton wool.
3. Set up the apparatus as shown in Figure 7.3.
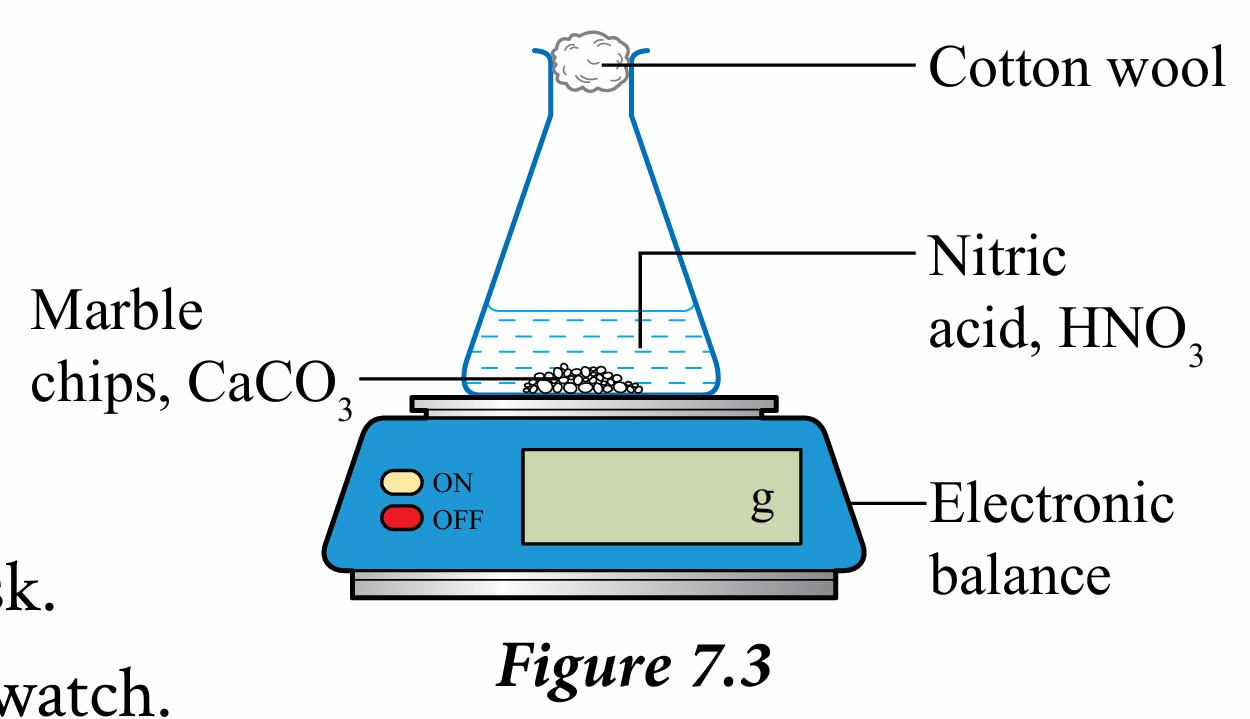
4. Add 10 g of marble chips, CaCO3 into the conical flask.
5. Immediately close the conical flask and start the stopwatch.
6. Record the reading of the electronic balance at intervals of 30 seconds.
7. Observe the changes that occur in the conical flask and record all observations.
8. Record your data in a table.
Discussion:
1. State the observable changes that was recorded in the activity.
2. Why does such a change occur? Explain your answer with the aid of a suitable chemical equation.
3. How would you know that the reaction is completed?
Answer:
Discussion:
1. Reduction in the mass of marble, CaCO3 (the weighing scale reading decreases).
2. Loss of carbon dioxide gas produced.
CaCO3(s) + 2HNO3(aq) → Ca(NO3)2(aq) + H2O(l) + CO2(g)
3. Effervescence stopped/ No gas bubbles are formed / No change in mass
Procedure:
1. Put 100 cm3 of 2.0 mol dm–3 nitric acid, HNO3 into a conical flask.
2. Close the mouth of the conical flask loosely with cotton wool.
3. Set up the apparatus as shown in Figure 7.3.

4. Add 10 g of marble chips, CaCO3 into the conical flask.
5. Immediately close the conical flask and start the stopwatch.
6. Record the reading of the electronic balance at intervals of 30 seconds.
7. Observe the changes that occur in the conical flask and record all observations.
8. Record your data in a table.
Discussion:
1. State the observable changes that was recorded in the activity.
2. Why does such a change occur? Explain your answer with the aid of a suitable chemical equation.
3. How would you know that the reaction is completed?
Answer:
Discussion:
1. Reduction in the mass of marble, CaCO3 (the weighing scale reading decreases).
2. Loss of carbon dioxide gas produced.
CaCO3(s) + 2HNO3(aq) → Ca(NO3)2(aq) + H2O(l) + CO2(g)
3. Effervescence stopped/ No gas bubbles are formed / No change in mass
(C) Reaction between potassium iodide solution, KI and lead(II) nitrate solution, Pb(NO3)2 :
Procedure:
1. Using separate weighing bottles, weigh 2 g of potassium iodide powder, KI and 2 g of lead(II) nitrate powder, Pb(NO3)2 by using two different bottles.
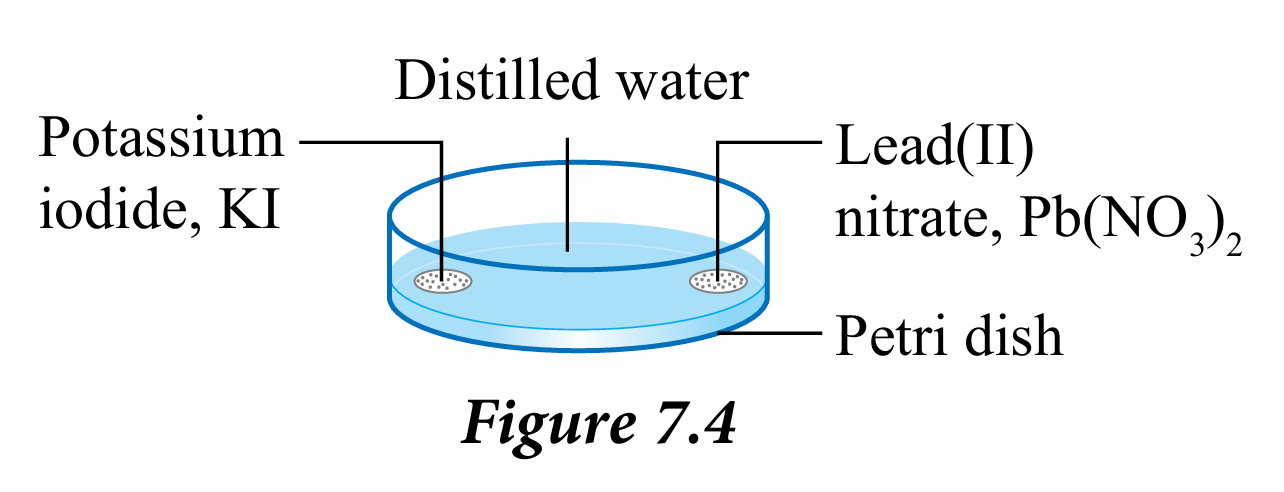
2. Pour distilled water into the petri dish to a depth of 0.5 cm.
3. Add the potassium iodide powder, KI into the water at the edge of the petri dish.
4. Add lead(II) nitrate powder, Pb(NO3)2 diagonally across from the potassium iodide powder, KI as shown in Figure 7.4.
5. Start the stopwatch immediately.
6. Record the time when the reaction is completed, that is, when no more precipitate is formed.
7. Filter the contents in the petri dish and wash the precipitate with distilled water.
8. Dry and weigh the precipitate.
9. Record your data in a table.
Discussion:
1. What is the colour of the precipitate formed?
2. Write an equation for the reaction that occurred.
Answer:
Discussion:
1. Yellow precipitate
2. Pb(NO3)2 (aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
Procedure:
1. Using separate weighing bottles, weigh 2 g of potassium iodide powder, KI and 2 g of lead(II) nitrate powder, Pb(NO3)2 by using two different bottles.
2. Pour distilled water into the petri dish to a depth of 0.5 cm.
3. Add the potassium iodide powder, KI into the water at the edge of the petri dish.
4. Add lead(II) nitrate powder, Pb(NO3)2 diagonally across from the potassium iodide powder, KI as shown in Figure 7.4.

5. Start the stopwatch immediately.
6. Record the time when the reaction is completed, that is, when no more precipitate is formed.
7. Filter the contents in the petri dish and wash the precipitate with distilled water.
8. Dry and weigh the precipitate.
9. Record your data in a table.
Discussion:
1. What is the colour of the precipitate formed?
2. Write an equation for the reaction that occurred.
Answer:
Discussion:
1. Yellow precipitate
2. Pb(NO3)2 (aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
