General Formula: CnH2n, n = 2, 3, 4, ….
Functional Group:
Double Bond
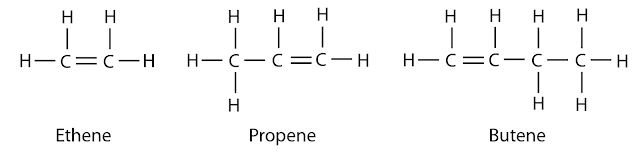
First 3 Members:
Chemical Properties:
MUST Know!
- Alkenes are unsaturated hydrocarbons.
- Alkenes are a family of hydrocarbons (compounds containing carbon and hydrogen only) containing a carbon-carbon double bond. Therefore, alkenes are unsaturated hydrocarbons.
- The general formula for alkene is CnH2n where n = 2, 3, ….
- There is no single carbon alkene because it need at least 2 carbon to form the double bond. Hence “methene” doesn’t exist.
Naming Alkene
- As alkane, the name of straight-chain alkenes is also made up of two component parts, the stem and the suffix.
- We use the same code for the stem, as the alkane.
- The suffix for alkene is “ene”.
- The table below shows the molecular formula and name of the first six alkenes.
| Formula | Name |
| C2H4 | Ethene |
| C3H6 | Propene |
| C4H8 | Butene |
| C5H10 | Pentene |
| C6H12 | Hexene |
| C7H14 | Heptene |