Activity 7.3:
Solving numerical problems related to rate of reactions
In the presence of manganese(IV) oxide, MnO2, hydrogen peroxide, H2O2 decomposes to water and oxygen. The oxygen gas released is collected in a gas syringe and the volume recorded at intervals of 0.5 minute. The data collected is shown in Table 7.3.
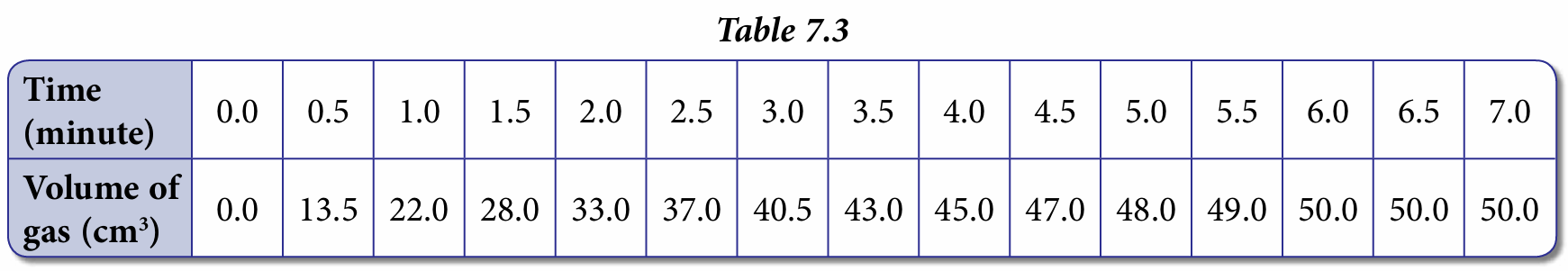
1. Based on Table 7.3, plot a graph of volume of gas against time.
2. Calculate the following average rate of reaction:
(a) For the first minute
(b) For the fifth minute
(c) For the whole reaction
3. Calculate the rate of reaction at the following time:
(a) 1.5 minute
(b) 4.0 minute
Answer:
1.
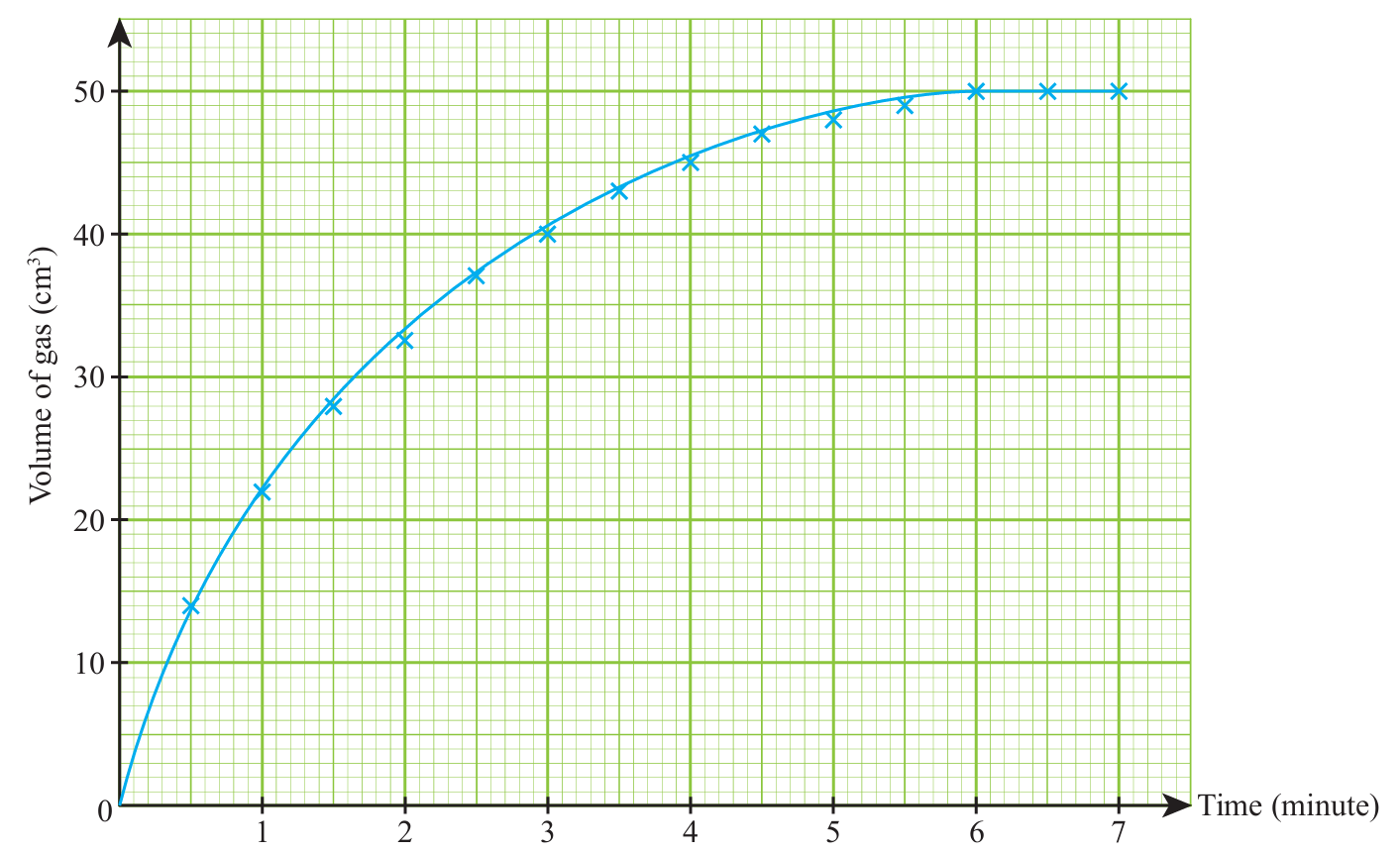
2.
(a)$$ \frac{(22-0) \mathrm{cm}^3}{(1-0) \mathrm{min}}=22 \mathrm{~cm}^3 \mathrm{~min}^{-1} $$
(b) $$ \frac{(48-45) \mathrm{cm}^3}{(5-4) \mathrm{min}}=3 \mathrm{~cm}^3 \mathrm{~min}^{-1} $$
(c) $$ \frac{50 \mathrm{~cm}^3}{6 \mathrm{~min}}=8.33 \mathrm{~cm}^3 \mathrm{~min}^{-1} $$
3.
(a) 10.62 cm3 min–1
(b) 4.17 cm3 min–1
Solving numerical problems related to rate of reactions
In the presence of manganese(IV) oxide, MnO2, hydrogen peroxide, H2O2 decomposes to water and oxygen. The oxygen gas released is collected in a gas syringe and the volume recorded at intervals of 0.5 minute. The data collected is shown in Table 7.3.

1. Based on Table 7.3, plot a graph of volume of gas against time.
2. Calculate the following average rate of reaction:
(a) For the first minute
(b) For the fifth minute
(c) For the whole reaction
3. Calculate the rate of reaction at the following time:
(a) 1.5 minute
(b) 4.0 minute
Answer:
1.

2.
(a)$$ \frac{(22-0) \mathrm{cm}^3}{(1-0) \mathrm{min}}=22 \mathrm{~cm}^3 \mathrm{~min}^{-1} $$
(b) $$ \frac{(48-45) \mathrm{cm}^3}{(5-4) \mathrm{min}}=3 \mathrm{~cm}^3 \mathrm{~min}^{-1} $$
(c) $$ \frac{50 \mathrm{~cm}^3}{6 \mathrm{~min}}=8.33 \mathrm{~cm}^3 \mathrm{~min}^{-1} $$
3.
(a) 10.62 cm3 min–1
(b) 4.17 cm3 min–1
