Experiment 7.2:
Aim: To investigate the effect of concentration of reactants on the rate of reaction.
Problem statement: How does the concentration of the reactants affect the rate of reaction?
Hypothesis: The higher the concentration of sodium thiosulphate solution, Na2S2O3, the shorter the time taken for the ‘X’ mark to disappear from view.
Variables:
(a) Manipulated : Concentration of sodium thiosulphate solution, Na2S2O3
(b) Responding : Time taken for the ‘X’ mark to disappear from view
(c) Fixed: Temperature, total volume of mixture, concentration and volume of sulphuric acid, H2SO4 and the size of the conical flask
Materials: 1.0 mol dm–3 sulphuric acid, H2SO4, 0.2 mol dm–3 sodium thiosulphate solution, Na2S2O3, distilled water and white piece of paper with a ‘X’ mark at the centre
Apparatus: 150 cm3 conical flask, stopwatch, 10 cm3 and 50 cm3 measuring cylinders
Procedure:
1. Put 45 cm3 of 0.2 mol dm–3 sodium thiosulphate solution, Na2S2O3 into a conical flask.
2. Place the conical flask on the ‘X’ mark on the white paper as shown in Figure 7.14.
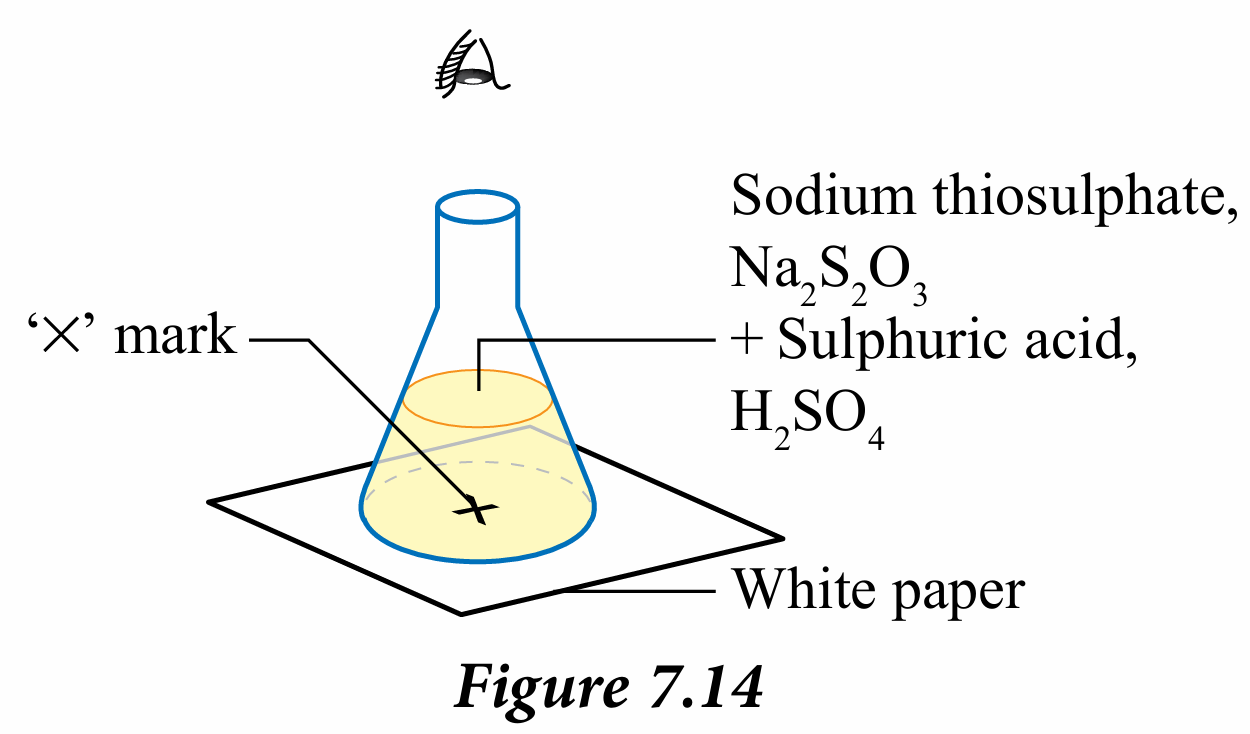
3. Swiftly, pour 5 cm3 of 1.0 mol dm–3 sulphuric acid, H2SO4 into the conical flask carefully and at the same time start the stopwatch.
4. Swirl the conical flask gently and place it again on the ‘X’ mark.
5. Observe the ‘X’ mark vertically from the mouth of the conical flask.
6. Stop the stopwatch once the ‘X’ mark disappears from view. Record the time taken.
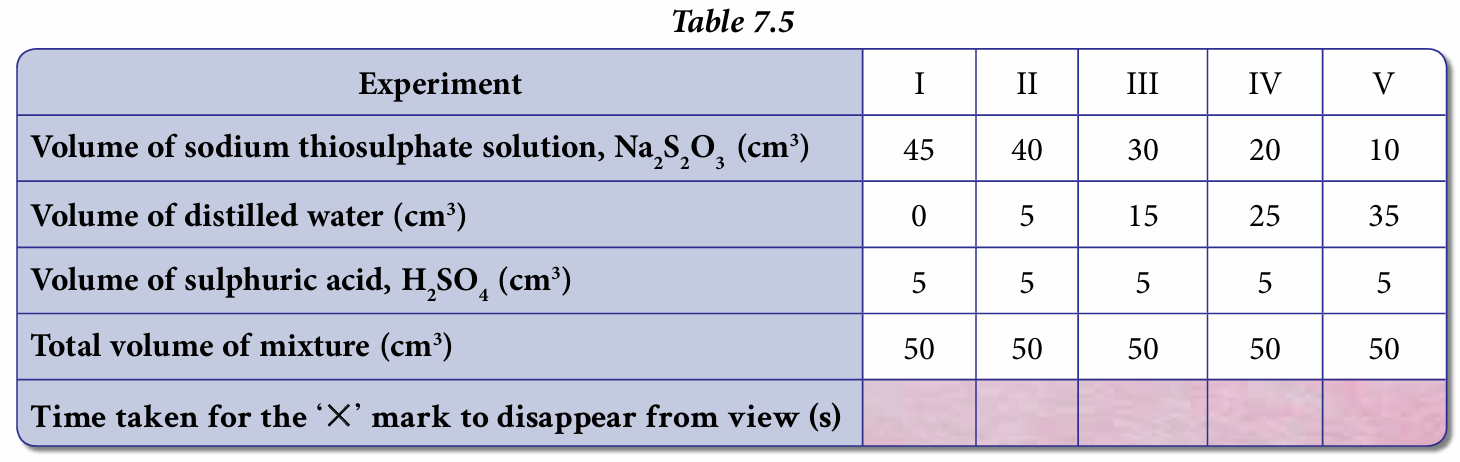
7. Repeat the experiment by using 0.2 mol dm–3 sodium thiosulphate solution, Na2S2O3 that has been diluted with distilled water as given in Table 7.5. The volume of 1.0 mol dm–3 sulphuric acid, H2SO4 is fixed at 5 cm3
8. Record all data in Table 7.5.
Results:
Interpreting data:
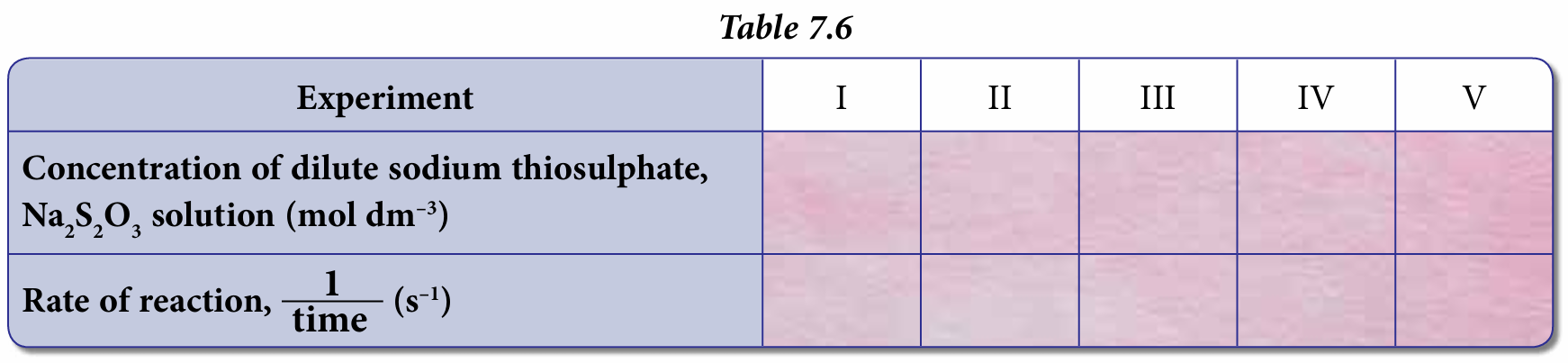
1. The concentration of dilute sodium thiosulphate solution, Na2S2O3 is calculated using the formula M1V1 = M2V2.

Use the given formula and the data collected to calculate the concentration of the dilute sodium thiosulphate solution, Na2S2O3.
2. In the experiment, the rate of reaction is inversely proportional to the time taken for the ‘X’ mark to disappear from view. Thus, rate of reaction = 1/time . Use this formula and the data collected to calculate the rate of reaction for all the five experiments.
3. Record all answers from (1) and (2) in Table 7.6.
4. Use the data in Table 7.6 to plot a graph of rate of reaction, 1/time against concentration of sodium thiosulphate solution, Na2S2O3, M2.
5. Based on the graph, state the relationship between rate of reaction and concentration of sodium thiosulphate solution, Na2S2O3.
Conclusion:
Is the hypothesis acceptable? What is the conclusion of this experiment?
Discussion:
1. Why does the solution in the conical flask turn cloudy?
2. Name the substance that causes the solution to turn cloudy.
3. The ‘X’ mark disappears from view when the solution in the conical flask reaches a certain level of cloudiness. What are the steps required in this experiment so that the same level of cloudiness is achieved in all the five experiments?
4. What are the changes being measured in the experiment to determine the rate of reaction?
Answer:
Discussion:
1. Formation of an insoluble solid in water.
2. Sulphur
3.
• The total volume of the solution is maintained.
• The height of the solution in the conical flask is maintained (use the same conical flask).
• Use a white paper with the same ‘X’ mark.
4. The time taken for a fixed mass of sulphur to be formed.
Aim: To investigate the effect of concentration of reactants on the rate of reaction.
Problem statement: How does the concentration of the reactants affect the rate of reaction?
Hypothesis: The higher the concentration of sodium thiosulphate solution, Na2S2O3, the shorter the time taken for the ‘X’ mark to disappear from view.
Variables:
(a) Manipulated : Concentration of sodium thiosulphate solution, Na2S2O3
(b) Responding : Time taken for the ‘X’ mark to disappear from view
(c) Fixed: Temperature, total volume of mixture, concentration and volume of sulphuric acid, H2SO4 and the size of the conical flask
Materials: 1.0 mol dm–3 sulphuric acid, H2SO4, 0.2 mol dm–3 sodium thiosulphate solution, Na2S2O3, distilled water and white piece of paper with a ‘X’ mark at the centre
Apparatus: 150 cm3 conical flask, stopwatch, 10 cm3 and 50 cm3 measuring cylinders
Procedure:
1. Put 45 cm3 of 0.2 mol dm–3 sodium thiosulphate solution, Na2S2O3 into a conical flask.
2. Place the conical flask on the ‘X’ mark on the white paper as shown in Figure 7.14.

3. Swiftly, pour 5 cm3 of 1.0 mol dm–3 sulphuric acid, H2SO4 into the conical flask carefully and at the same time start the stopwatch.
4. Swirl the conical flask gently and place it again on the ‘X’ mark.
5. Observe the ‘X’ mark vertically from the mouth of the conical flask.
6. Stop the stopwatch once the ‘X’ mark disappears from view. Record the time taken.
7. Repeat the experiment by using 0.2 mol dm–3 sodium thiosulphate solution, Na2S2O3 that has been diluted with distilled water as given in Table 7.5. The volume of 1.0 mol dm–3 sulphuric acid, H2SO4 is fixed at 5 cm3
8. Record all data in Table 7.5.
Results:

Interpreting data:
1. The concentration of dilute sodium thiosulphate solution, Na2S2O3 is calculated using the formula M1V1 = M2V2.

Use the given formula and the data collected to calculate the concentration of the dilute sodium thiosulphate solution, Na2S2O3.
2. In the experiment, the rate of reaction is inversely proportional to the time taken for the ‘X’ mark to disappear from view. Thus, rate of reaction = 1/time . Use this formula and the data collected to calculate the rate of reaction for all the five experiments.
3. Record all answers from (1) and (2) in Table 7.6.

4. Use the data in Table 7.6 to plot a graph of rate of reaction, 1/time against concentration of sodium thiosulphate solution, Na2S2O3, M2.
5. Based on the graph, state the relationship between rate of reaction and concentration of sodium thiosulphate solution, Na2S2O3.
Conclusion:
Is the hypothesis acceptable? What is the conclusion of this experiment?
Discussion:
1. Why does the solution in the conical flask turn cloudy?
2. Name the substance that causes the solution to turn cloudy.
3. The ‘X’ mark disappears from view when the solution in the conical flask reaches a certain level of cloudiness. What are the steps required in this experiment so that the same level of cloudiness is achieved in all the five experiments?
4. What are the changes being measured in the experiment to determine the rate of reaction?
Answer:
Discussion:
1. Formation of an insoluble solid in water.
2. Sulphur
3.
• The total volume of the solution is maintained.
• The height of the solution in the conical flask is maintained (use the same conical flask).
• Use a white paper with the same ‘X’ mark.
4. The time taken for a fixed mass of sulphur to be formed.
