Oxidising Agents and Reduction Agents
- In a redox reaction, a compound that is reduced is the oxidizing agent. An oxidising agent is a substance which oxidises something else.
- Inversely, a compound that is oxidised is the reducing agent. A reducing agent reduces something else.
Example:
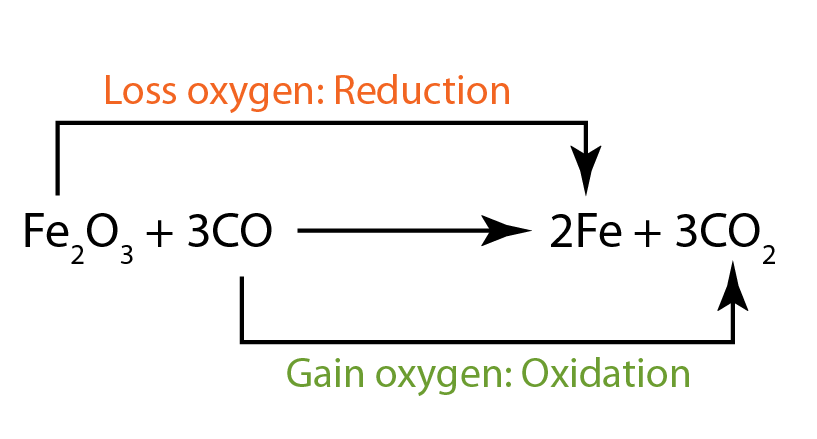
- In this reaction, iron(III) oxide is reduced. Therefore it is the oxidising agent. It has oxidised carbon monoxide to become carbon dioxide.
- The carbon monoxide is oxidised. Therefore it acts as the reducing agent. It has reduced iron(III) oxide to become iron metal.
Example:
In this reaction:
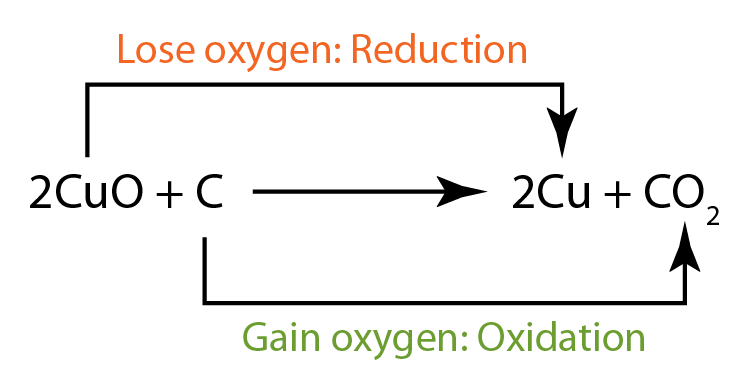
- copper(II) oxide is reduced, hence it is the oxidising agent.
- carbon is oxidised, hence it is the reducing agent.
Commonly Used Oxidising and Reducing Agent and Their Half Equation
Below are the oxidising and reducing agent that normaly in use
Oxidising Agent
Acided Potassium Manganate (VII)
Observation:
Acided Potassium Dicromate (VI)
Observation:
Hydrogen Peroxide
Concentrated Nitric Acid
Reducing Agent
Sulphur Dioxide
Hydrogen Sulphide
Sodium Sulphite Aqueous
Tin(II) Chloride Aqueous
Forming Ionic Equation from the Half Equation
- Ionic equation of a redox reaction can be formed from the half equations of the reaction.
- When writing the ionic equation, make sure that the number of electrons in both the oxidation reaction and reduction reaction is balanced.
Example:
Redox reaction between potassium iodide and potassium manganate (VII)
Half equations
2I– → I2 + 2e ——-(1)
MnO4– + 8H+ + 5e → Mn2+ + 4H2O ——-(2)
To make the number of electrons in both chemical equation equal
(1) x 5
(2) x 2
Ionic Equations
Add the 2 equations together. Exclude the electrons.
Example:
Redox reaction between iron(II) sulphate and potassium dicromate(VI)
Half equations
Fe2+ → Fe3+ + e ——-(1)
Cr2O72- + 14H+ + 6e → 2Cr3+ + 7H2O ——-(2)
To make the number of electrons in both chemical equation equal
(1) x 5
(2)
Ionic Equations
Add the 2 equations together. Exclude the electrons.
Example:
Redox reaction between iron(III) nitrate and sulphur dioxide gas
Half equations
Fe3+ → Fe2+ + e ——-(1)
SO2 + 2H2O → SO42- + 4H+ + 2e ——-(2)
To make the number of electrons in both chemical equation equal
(1) x 2
(2)
Ionic Equations
Add the 2 equations together. Exclude the electrons.
Example:
Redox reaction between iron(III) chloride and hydrogen sulphide gas
Half equations
Fe3+ → Fe2+ + e ——-(1)
H2S → 2H+ + S + 2e ——-(2)
To make the number of electrons in both chemical equation equal
(1) x 2
H2S → 2H+ + S + 2e
Ionic Equations
Add the 2 equations together. Exclude the electrons.