Position of Hydrogen in The Reactivity Series of Metals
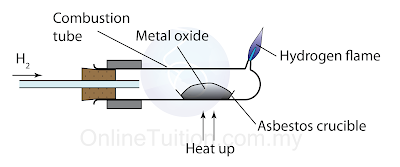
- The position of hydrogen in the reactivity series of metal can also be determined based on its ability to displace oxygen from metal oxides.
- If hydrogen is more reactive than a metal, it can displace oxygen from metal oxide, and reduces the metal oxide to its metal.
- Hydrogen + metal oxide → metal + water
- Conversely, if hydrogen cannot remove oxygen from metal oxide, hydrogen is less reactive than the metal in the reactivity series of metal.
- Hydrogen can reduce iron (II) oxide, Fe2O3 to form iron, Fe and water.Fe2O3 + 3H2 → 2Fe + 3H2O
- However, hydrogen cannot reduce zinc oxide, ZnO.
- Therefore hydrogen is below zinc but above iron in the reactivity series of metals.
- The diagram below shows the set-up of apparatus used to determine the position of hydrogen in the reactivity series of metal.
- The chart below shows the position of carbon and hydrogen in the reactivity series of metal base on their ability to attract oxygen to form oxide.