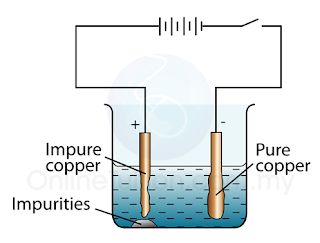
Industrial Applications of Electrolysis – Purifying of Copper
- Copper is a good electrical conductor and is used extensively to make electrical wiring and components. However, the presence of impurity in copper can reduce its electrical conductivity.
- In industry, electrolysis processed is used to purify copper, and the process is called electrolytic refining.
Electrolytic Refining of Copper
- Figure above shows the illustration of the apparatus setup to investigate electrolytic refining of copper.
- When electricity flows, the copper in the impure anode dissolves in the solution to form copper ions.
- Copper ions are then deposit on the cathode which consist of a piece of pure copper.
- In SPM, you need to know
- the electrolyte used
- the electrode for the impure and pure copper
- the reaction at anode and cathode
Electrolyte:
Copper(II) sulphate
CuSO4 à Cu2++ SO42-
Electrode:
Anode: Impure copper
Cathode: Pure copper
Chemical Reaction
Anode:
Cu —> Cu2+ + 2e
In anode, the copper atoms from the electrode are ionised to form copper(II) ions.
Cathode
Cu2+ —> Cu + 2e
In cathode, the copper ions are discharged to form copper atom and then deposit on the surface of the key
Note: Impurities in the copper do not dissolve, and instead fall off the anode as anode sludge. At the cathode, the copper ions are deposited as pure copper metal.