Industrial Applications of Electrolysis – Electroplating
- Electroplating is a process to coat an object with a thin protective layer of metal
- Electroplating is used to
- prevent corrosion
- improve the appearance of the objects
- In electroplating,
- the anode is the electroplating metal
- the cathode is the object to be electroplated
- the electrolyte must contain the ions of the plating meta
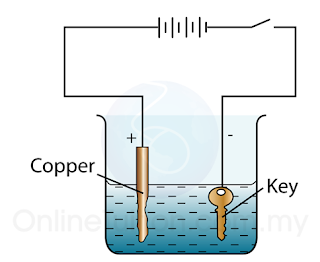
Example: Electroplate a Key with Copper
Electrolyte:
Copper(II) sulphate
CuSO4 —> Cu2+ + SO42-
Electrode:
Anode: Copper
Cathode: Object to be electroplated
Chemical Reaction
Anode:
Cu —> Cu2+ + 2e
In anode, the copper atoms from the electrode are ionised to form copper(II) ions.
Note: The anode is then made of the metal we wish to plate with (copper), and the electrolyte needs to be a solution of a salt of this metal (copper(II) sulphate).
Cathode
Cu2+ —> Cu + 2e
In cathode, the copper ions are discharged to form copper atom and then deposit on the surface of the key
Note: we need to make the cathode the object for plating