Factors Affecting the Selective Discharge – Concentration
If the concentration of a particular ion is high, it may be selected to be discharged even though it is higher in the electrochemical series compares with another ion present in the solution.
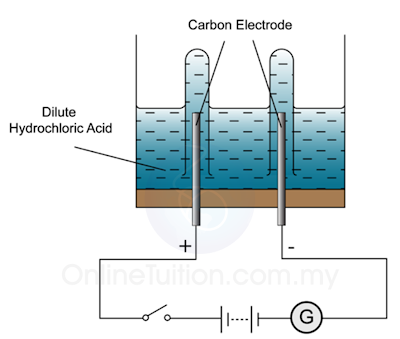
Example
| Electrolysis of Diluted Hydrochloric Acid | Electrolysis of Concentrated Hydrochloric Acid |
| Ions presence at Anode: Cl–, OH– Cathode: H+ | Ions presence at Anode: Cl–, OH– Cathode: H+ |
| Observation at Anode: Colour gas is produced. When a glowing wooden splinter is inserted into the test tube that contain the gas, the splinter is rekindled. Cathode | Observation at Anode: A greenish yellow gas produced. When a blue litmus paper is inserted into the test tube that contain the gas, the blue litmus paper turn red and then become colourless. Cathode |
| Half Equation of the Reaction at Anode: 4OH– → 2H2O + O2 + 4e Cathode: H+ + 2e → H2 | Half Equation of the Reaction at Anode: 2Cl– → Cl + 2e Cathode: H+ + 2e → H2 |
Note: At anode, the position of hydroxide ion (OH–) is lower compare to chloride ion (Cl–). However, chloride is selected to be discharged because its concentration is much higher than he concentration of hydroxide ion.
Additional Note:
- Nevertheless, concentration is not a determining factor. It only affect the selective discharge of the ions which is very close to each other in electrochemical series.
- The chart below summurises the ions that likely to be selected if they are presence with hydrogen ions at cathode or hydroxide ions at anode with high concentration.