Physical Properties of Halogens
- All group 17 elements are non-metals. Therefore they are heat and electricity insulator.
- The table below shows the electron arrangement and physical properties of group 17 elements.
| Name | Colour | Melting point | Boiling point |
| Fluorine | pale yellow gas | -220ºC | -188ºC |
| Chlorine | Yellowish green gas | -102ºC | -34ºC |
| Bromine | dark red liquid, brown vapour | -7ºC | 59ºC |
| Iodine | black solid, purple vapour | 114ºC | 184ºC |
| Astatine | black solid, dark vapour | 302ºC | 380ºC |
Size of Atom and Density
- The atomic size of group 17 elements increases down the group.
- This is due to the increase of the number of electron shell down the group.
- The density of group 17 elements also increase down the group.
- This is because the rate of increment of the atomic mass is higher than the rate of increment of the volume.
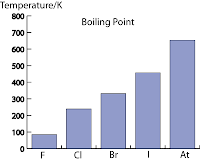
Melting Point and Boiling Point
- As shown in the graph to the right, the melting points and boiling increase steadily down the group.
- The physical state at room temperature also change from gas to liquid and then to solid.
- This is because of the intermolecular attractive force (van der Waals force) increase with increasing size of atom or molecule.