Experiment 8.1:
Aim: To investigate the comparison between the properties of alloys and pure metals.
Problem statement: Is an alloy more resistant to corrosion and harder than a pure metal?
Materials: Stainless steel plate, iron plate, distilled water, bronze block and copper block
Apparatus: 100 cm3 beaker, 100 cm3 measuring cylinder, sandpaper, steel ball bearing, 1 kg weight, retort stand with clamp, meter ruler and cellophane tape
(A) Resistance to corrosion
Hypothesis: Stainless steel is more resistant to corrosion than iron.
Variables:
(a) Manipulated : Type of plate
(b) Responding : Corrosion of plate
(c) Fixed : Size of plate and volume of distilled water
Procedure:
1. Clean the surfaces of stainless steel and iron plates by using a sandpaper. Observe the surfaces of both plates. Record your observations.
2. Immerse both plates in a beaker containing 80 cm3 of distilled water as shown in Figure 8.2.
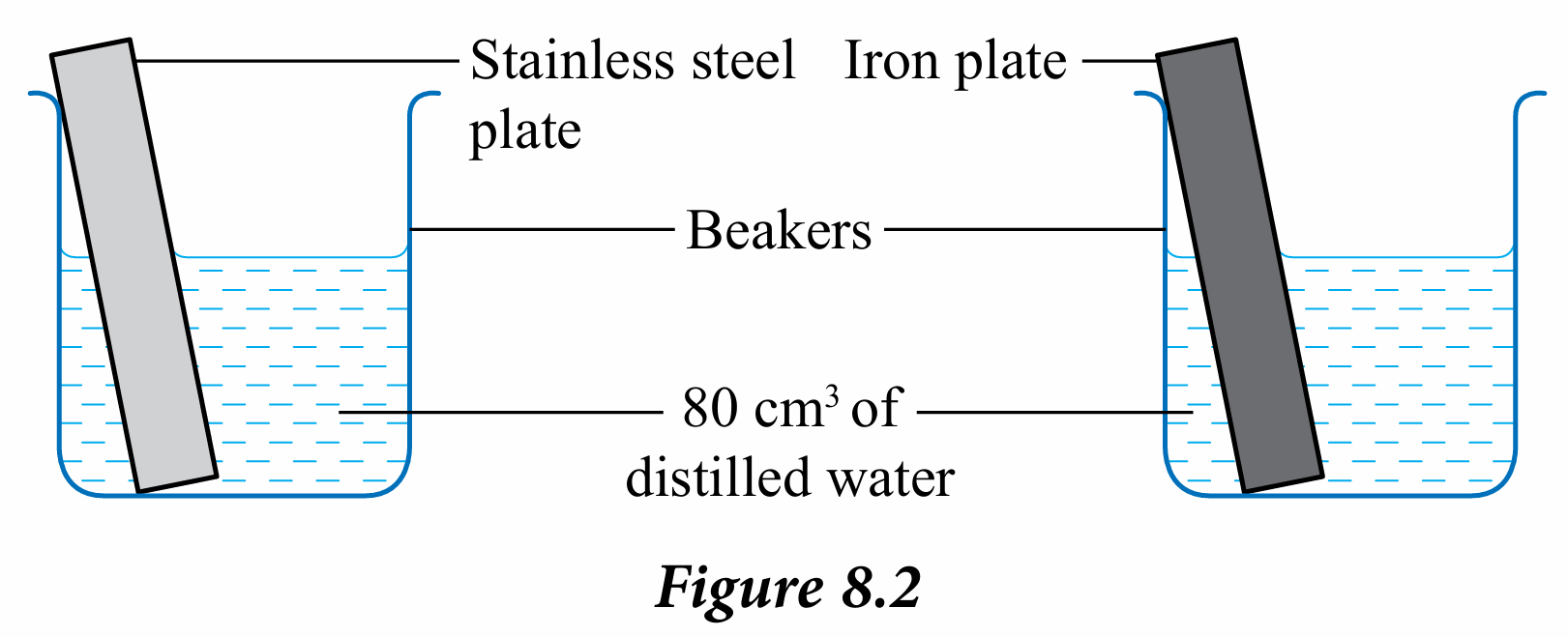
3. Leave both beakers aside for one week.
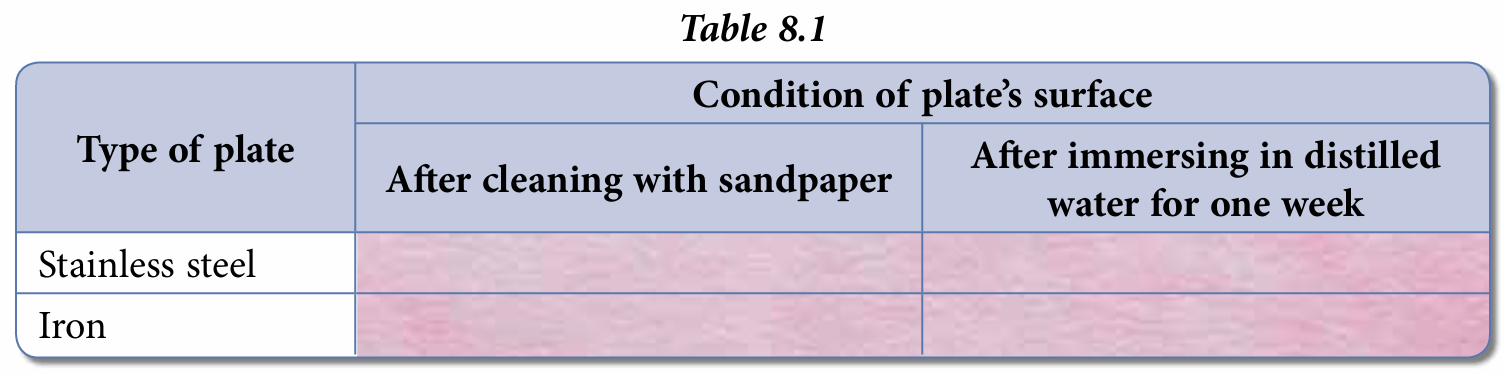
4. After one week, remove both plates and observe the conditions of their surfaces. Record your observations in Table 8.1.
Results:
(B) Hardness of substances
Hypothesis: Construct a suitable hypothesis for this experiment.
Variables: State the variables involved in this experiment.
Procedure:
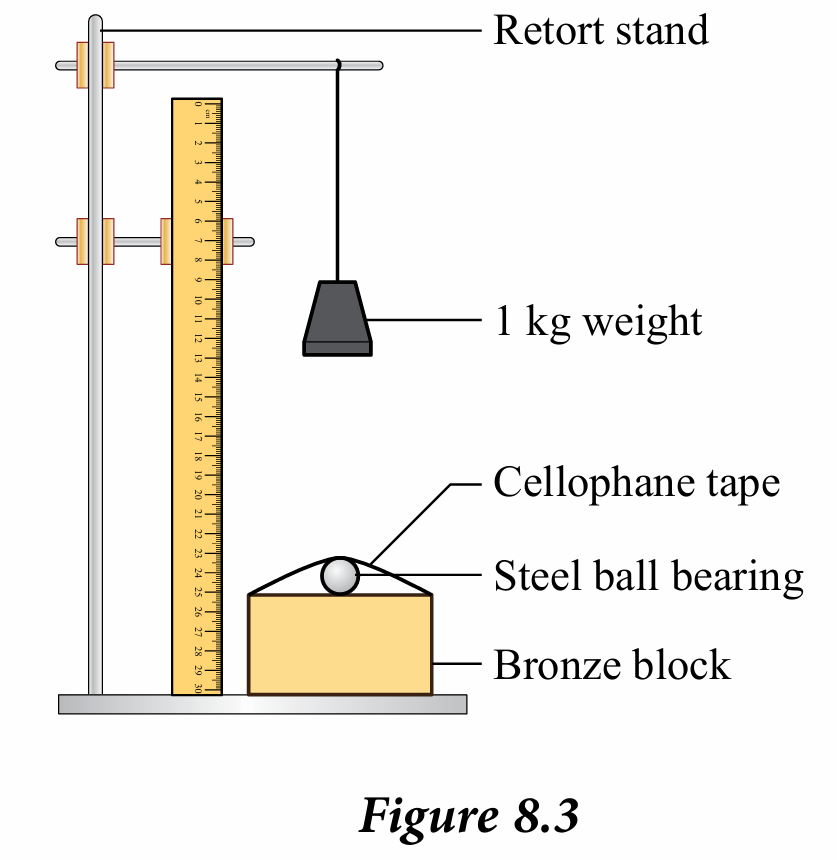
1. Fix a steel ball bearing on the surface of the bronze block using a cellophane tape.
2. Hang a 1 kg weight on the retort stand at 50 cm above the surface of the bronze block, as shown in Figure 8.3.
3. Release the weight onto the steel ball bearing.
4. Measure the diameter of the dent formed on the surface of the bronze block.
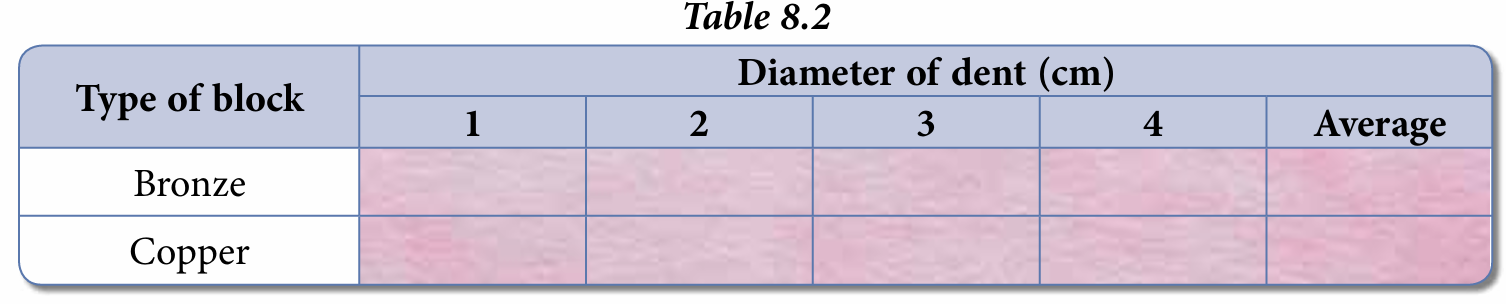
5. Repeat steps 1 to 4 three times but on different surfaces of the bronze block to obtain an average diameter of the dent formed. Record the reading in Table 8.2.
6. Repeat steps 1 to 5 by replacing the bronze block with a copper block.
Results:
Conclusion:
Is the hypothesis acceptable? What is the conclusion of this experiment?
Discussion:
1. Why must the stainless steel plate and iron plate be cleaned with a sandpaper?
2. Compare the rate of corrosion of the stainless steel and iron plates.
3. Which block formed a dent with a larger diameter?
4. State the relationship between the diameter of the dent and the hardness of the substance.
Answer:
Discussion:
1. To remove the oxide layer from the surface of the metal.
2. The rate of corrosion of stainless steel is lower compared to iron.
3. Copper block
4. The smaller the diameter of the dent, the harder the substance.
Aim: To investigate the comparison between the properties of alloys and pure metals.
Problem statement: Is an alloy more resistant to corrosion and harder than a pure metal?
Materials: Stainless steel plate, iron plate, distilled water, bronze block and copper block
Apparatus: 100 cm3 beaker, 100 cm3 measuring cylinder, sandpaper, steel ball bearing, 1 kg weight, retort stand with clamp, meter ruler and cellophane tape
(A) Resistance to corrosion
Hypothesis: Stainless steel is more resistant to corrosion than iron.
Variables:
(a) Manipulated : Type of plate
(b) Responding : Corrosion of plate
(c) Fixed : Size of plate and volume of distilled water
Procedure:
1. Clean the surfaces of stainless steel and iron plates by using a sandpaper. Observe the surfaces of both plates. Record your observations.
2. Immerse both plates in a beaker containing 80 cm3 of distilled water as shown in Figure 8.2.

3. Leave both beakers aside for one week.
4. After one week, remove both plates and observe the conditions of their surfaces. Record your observations in Table 8.1.
Results:

(B) Hardness of substances
Hypothesis: Construct a suitable hypothesis for this experiment.
Variables: State the variables involved in this experiment.
Procedure:

1. Fix a steel ball bearing on the surface of the bronze block using a cellophane tape.
2. Hang a 1 kg weight on the retort stand at 50 cm above the surface of the bronze block, as shown in Figure 8.3.
3. Release the weight onto the steel ball bearing.
4. Measure the diameter of the dent formed on the surface of the bronze block.
5. Repeat steps 1 to 4 three times but on different surfaces of the bronze block to obtain an average diameter of the dent formed. Record the reading in Table 8.2.
6. Repeat steps 1 to 5 by replacing the bronze block with a copper block.
Results:

Conclusion:
Is the hypothesis acceptable? What is the conclusion of this experiment?
Discussion:
1. Why must the stainless steel plate and iron plate be cleaned with a sandpaper?
2. Compare the rate of corrosion of the stainless steel and iron plates.
3. Which block formed a dent with a larger diameter?
4. State the relationship between the diameter of the dent and the hardness of the substance.
Answer:
Discussion:
1. To remove the oxide layer from the surface of the metal.
2. The rate of corrosion of stainless steel is lower compared to iron.
3. Copper block
4. The smaller the diameter of the dent, the harder the substance.
