Question 1:
The addition of coke (carbon) in the extraction process of iron is to remove oxygen from iron ore. The iron and carbon mixture will form steel. Table 1 shows two types of steel with different percentage of carbon.
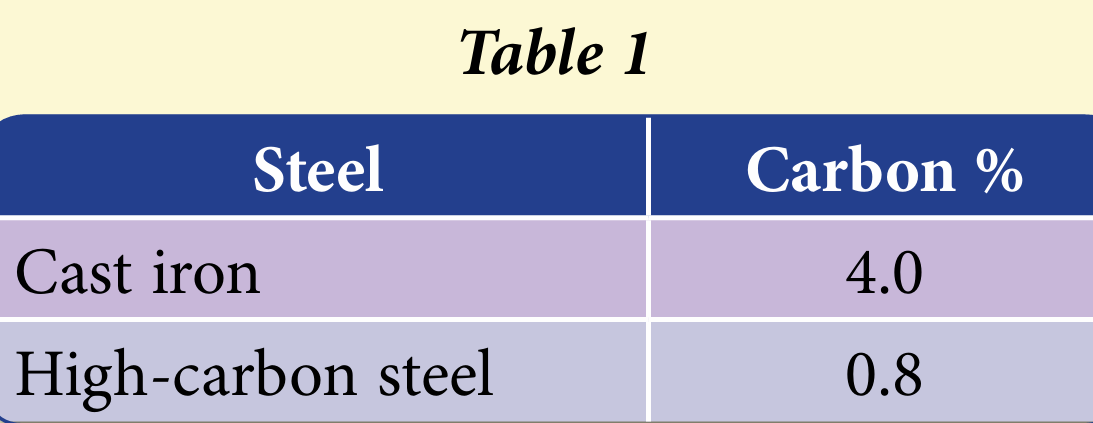
(a) Cast iron is brittle whereas high-carbon steel is hard and strong. Based on Table 1, calculate the percentage of carbon that must be removed from cast iron to produce high-carbon steel.
(b) Stainless steel is produced from a mixture of chromium, nickel and carbon.
(i) State the percentage of chromium, nickel and carbon in stainless steel.
(ii) Stainless steel is suitable to be used to make high quality knife blades. Explain.
Answer:
(a)
$$ \begin{aligned} \% \text { carbon that must be removed } & =\frac{(4.0-0.8)}{4.0} \times 100 \\ & =80 \% \end{aligned} $$
(b)(i) % chromium = 18%, % nickel = 8%, % carbon = 1%
(b)(ii) Stainless steel does not rust when it is in contact with water and oxygen
The addition of coke (carbon) in the extraction process of iron is to remove oxygen from iron ore. The iron and carbon mixture will form steel. Table 1 shows two types of steel with different percentage of carbon.

(a) Cast iron is brittle whereas high-carbon steel is hard and strong. Based on Table 1, calculate the percentage of carbon that must be removed from cast iron to produce high-carbon steel.
(b) Stainless steel is produced from a mixture of chromium, nickel and carbon.
(i) State the percentage of chromium, nickel and carbon in stainless steel.
(ii) Stainless steel is suitable to be used to make high quality knife blades. Explain.
Answer:
(a)
$$ \begin{aligned} \% \text { carbon that must be removed } & =\frac{(4.0-0.8)}{4.0} \times 100 \\ & =80 \% \end{aligned} $$
(b)(i) % chromium = 18%, % nickel = 8%, % carbon = 1%
(b)(ii) Stainless steel does not rust when it is in contact with water and oxygen
Question 2:
Lead crystal glass can be used to make spectacle lenses.
(a) What is the composition of lead crystal glass?
(b) Explain the advantages and disadvantages of using lead crystal glass to make spectacle lenses.
(c) Nowadays, spectacle lenses are made from polycarbonate polymer. The properties of polycarbonate are as follows:
• Low density and easily moulded
• Absorbs UV rays and is very transparent
• High impact resistance
You need a pair of new spectacles. Will you choose lenses made from lead crystal glass or polycarbonate? Explain your answer.
Answer:
(a) Silica, SiO2; soda (Na2CO3); lead(II) oxide (PbO)
(b)
Advantages:
• Transparent / high refractive index
Disadvantages:
• High density / break easily (brittle) / low resistance to scratching / does not absorb UV rays
(c)
Polycarbonate lens:
• Light
• Does not break easily when dropped
• Many attractive / unique lens design
• Eye safety – protection from UV rays
• High clarity
Lead crystal glass can be used to make spectacle lenses.
(a) What is the composition of lead crystal glass?
(b) Explain the advantages and disadvantages of using lead crystal glass to make spectacle lenses.
(c) Nowadays, spectacle lenses are made from polycarbonate polymer. The properties of polycarbonate are as follows:
• Low density and easily moulded
• Absorbs UV rays and is very transparent
• High impact resistance
You need a pair of new spectacles. Will you choose lenses made from lead crystal glass or polycarbonate? Explain your answer.
Answer:
(a) Silica, SiO2; soda (Na2CO3); lead(II) oxide (PbO)
(b)
Advantages:
• Transparent / high refractive index
Disadvantages:
• High density / break easily (brittle) / low resistance to scratching / does not absorb UV rays
(c)
Polycarbonate lens:
• Light
• Does not break easily when dropped
• Many attractive / unique lens design
• Eye safety – protection from UV rays
• High clarity
