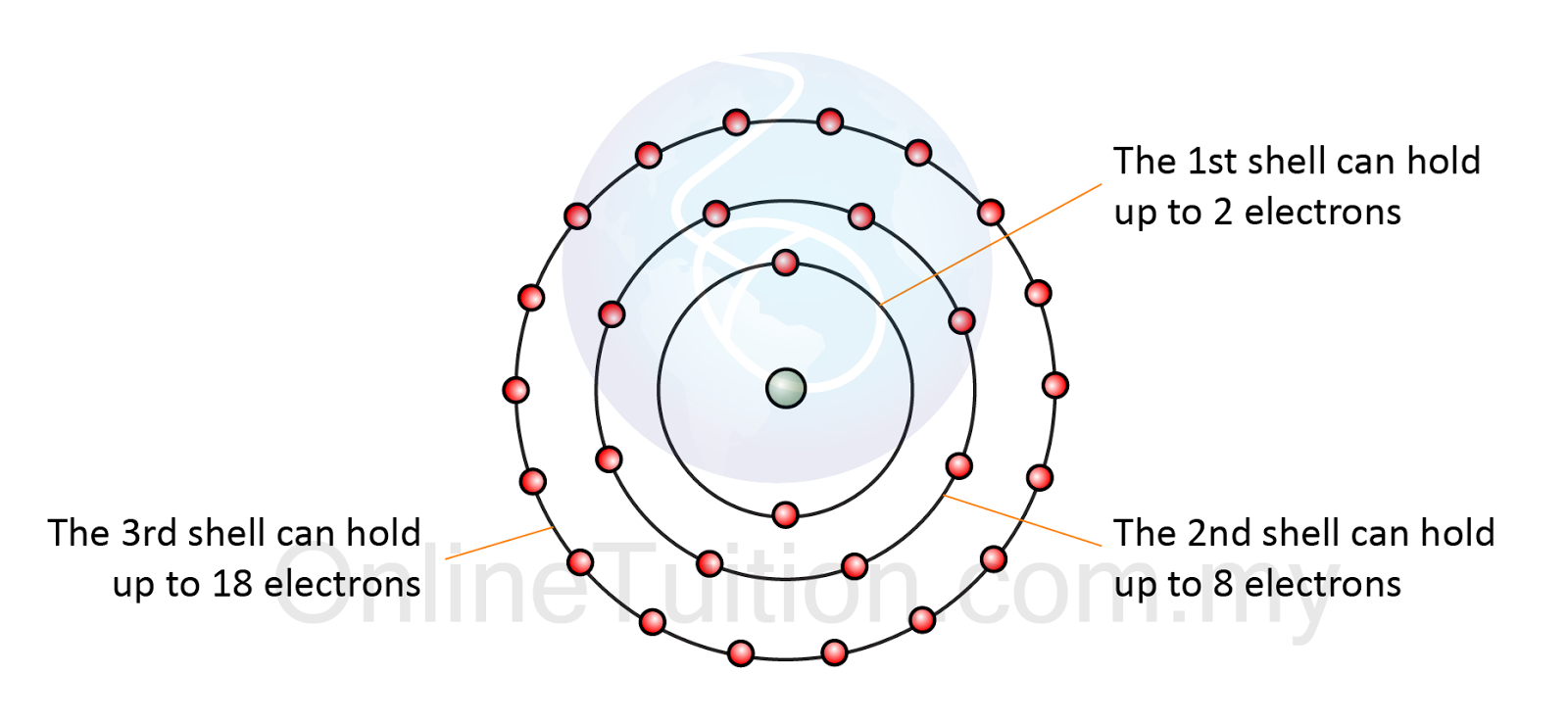
- We have learnt that electrons occupy orbits with definite energy level of an atom, as suggested by Neils Bohr.
- These orbits with definite energy level are known as the shell.
- Every single shell is capable of holding up to certain amount of electrons.
- The first shell can hold up to two electrons. This is called a duplet.
- The second shell can hold up to eight electrons. This is called an octet.
- The third shell can hold up to eighteen electrons.
- However, with the third shell, when eight electrons are present, extra stability is gained. The additional electrons go into the fourth shell before the third shell is completely filled.
- The way in which the electrons are distributed in the shells of an atom is called the electron arrangement of the atom
- The examples below show the electron arrangement of some elements:
|
Atom |
Notes |
Electrons Arrangement |
 |
|
2.1 |
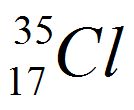 |
|
2.8.7 |
 |
|
2.8.8.2 |
| Element | Proton Number | Number of Electron |
Number of electron in |
Electron Arrangement | |||
| 1st shell | 2nd shell | 3rd shell | 4th shell | ||||
| Hydrogen |
1 |
1 |
1 |
0 |
0 |
0 |
1 |
| Helium |
2 |
2 |
2 |
0 |
0 |
0 |
2 |
| Lithium |
3 |
3 |
2 |
1 |
0 |
0 |
2.1 |
| Beryllium |
4 |
4 |
2 |
2 |
0 |
0 |
2.2 |
| Boron |
5 |
5 |
2 |
3 |
0 |
0 |
2.3 |
| Carbon |
6 |
6 |
2 |
4 |
0 |
0 |
2.4 |
| Nitrogen |
7 |
7 |
2 |
5 |
0 |
0 |
2.5 |
| Oxygen |
8 |
8 |
2 |
6 |
0 |
0 |
2.6 |
| Fluorine |
9 |
9 |
2 |
7 |
0 |
0 |
2.7 |
| Neon |
10 |
10 |
2 |
8 |
0 |
0 |
2.8 |
| Sodium |
11 |
11 |
2 |
8 |
1 |
0 |
2.8.1 |
| Magnesium |
12 |
12 |
2 |
8 |
2 |
0 |
2.8.2 |
| Aluminium |
13 |
13 |
2 |
8 |
3 |
0 |
2.8.3 |
| Silicon |
14 |
14 |
2 |
8 |
4 |
0 |
2.8.4 |
| Phosphorus |
15 |
15 |
2 |
8 |
5 |
0 |
2.8.5 |
| Sulphur |
16 |
16 |
2 |
8 |
6 |
0 |
2.8.6 |
| Chlorine |
17 |
17 |
2 |
8 |
7 |
0 |
2.8.7 |
| Argon |
18 |
18 |
2 |
8 |
8 |
0 |
2.8.8 |
| Potassium |
19 |
19 |
2 |
8 |
8 |
1 |
2.8.8.1 |
| Calcium |
20 |
20 |
2 |
8 |
8 |
2 |
2.8.8.2 |
Recommended Videos
Electron arrangement in an atom
Valence Electron
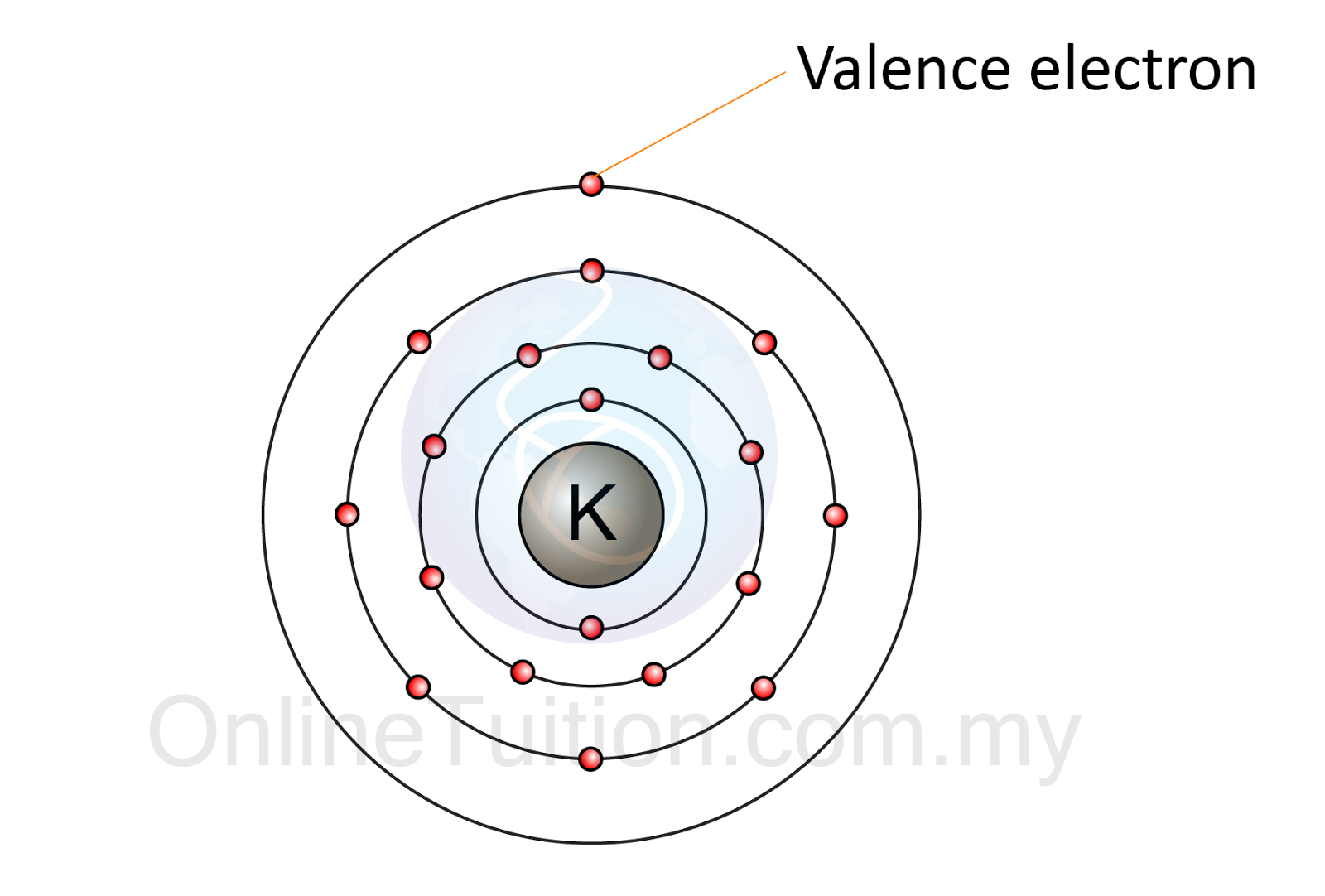
Valence electrons are the electrons in the outermost shell.
- The electrons in the outermost shell of an atom are called valence electrons.
- The valence electrons have great significance in determining the chemical properties of an atom.
- Elements with the same number of valence electron have the same chemical properties.
Example:
Given that a sodium atom has 11 protons 12 neutrons. Find the number of valence electron in a sodium atom.
Answer:
For an atom,
Number of electrons = number of protons = 11
Electron arrangement of sodium = 2.8.1
Therefore, sodium has 1 valence electron.
Menu