Question 3:
Traditional ceramics are made from clay such as kaolin.
(a) Name two oxide compounds found in kaolin.
(b) Give the formula of the ion that produces brown colour in clay.
(c) State two uses of traditional ceramics.
Answer:
(a) Silicon dioxide and aluminium oxide
(b) Fe3+ ion
(c) Manufacture of ceramics and bricks
Traditional ceramics are made from clay such as kaolin.
(a) Name two oxide compounds found in kaolin.
(b) Give the formula of the ion that produces brown colour in clay.
(c) State two uses of traditional ceramics.
Answer:
(a) Silicon dioxide and aluminium oxide
(b) Fe3+ ion
(c) Manufacture of ceramics and bricks
Question 4:
The various unique properties of ceramics are modified in its use in various fields. State the property of ceramic involved in the manufacture of the following objects:
(a) Car engine
(b) Spark plug
Answer:
(a) Heat insulator / light / resistant to corrosion
(b) Heat insulator / electrical insulator
The various unique properties of ceramics are modified in its use in various fields. State the property of ceramic involved in the manufacture of the following objects:
(a) Car engine
(b) Spark plug
Answer:
(a) Heat insulator / light / resistant to corrosion
(b) Heat insulator / electrical insulator
Question 5:
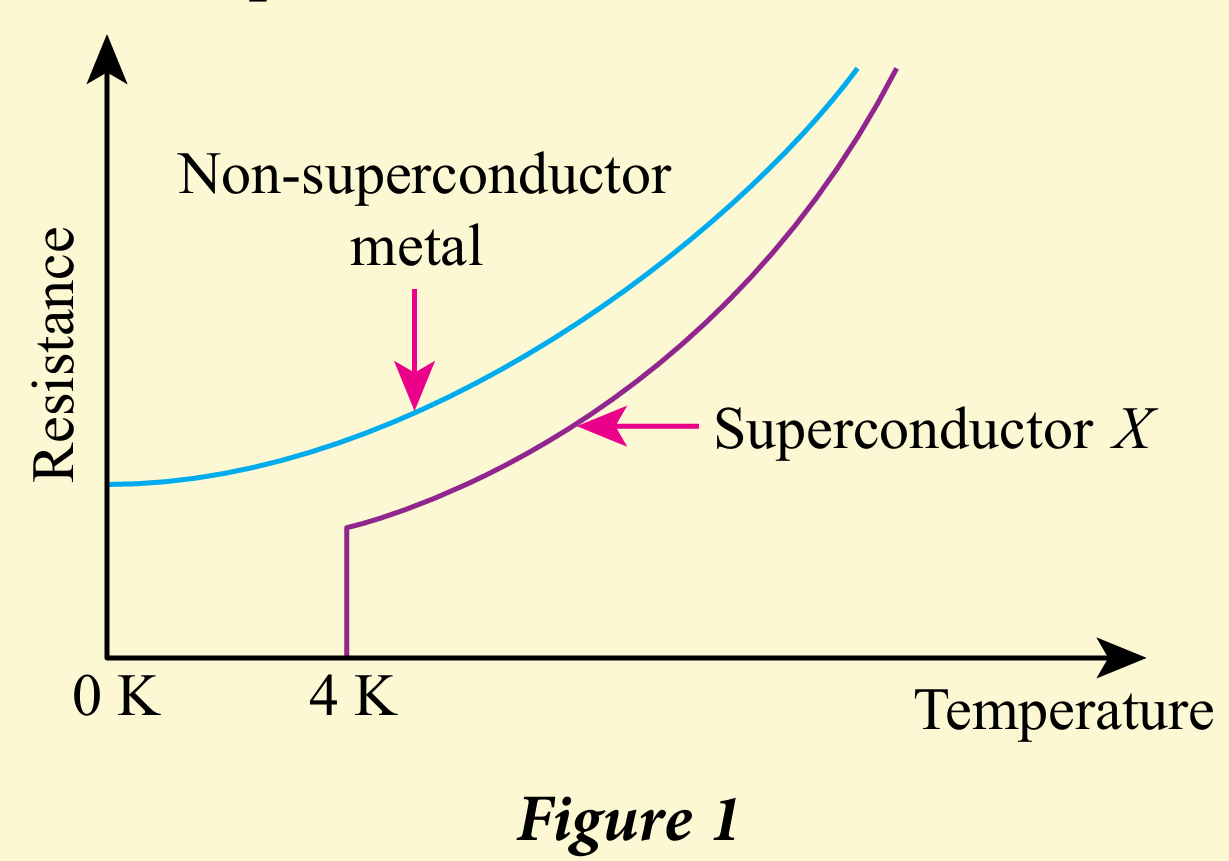
Metals can conduct electricity. Ceramic materials can also be processed to conduct electricity and be made superconductors. Figure 1 shows the change in the electrical resistance value of two conductors against the temperature.
(a) Give an example of a ceramic that shows superconductivity properties.
(b) Explain the difference in the electrical conductivity properties of the two conductors in Figure 1.
(c) How do scientists create a very cold condition to investigate the superconductivity phenomena?
Answer:
(a) Yttrium barium copper oxide ceramic, YBCO
(b) When temperature drops, the electrical resistance of metals that are not superconductors also drops but the resistance does not disappear even though at the lowest temperature 0 K When temperature drops, the electrical resistance of superconductor X also drops and the resistance disappears at a very low critical temperature (4 K) – close to absolute zero, 0 K.
(c) Cool the substance in liquid nitrogen or helium
Metals can conduct electricity. Ceramic materials can also be processed to conduct electricity and be made superconductors. Figure 1 shows the change in the electrical resistance value of two conductors against the temperature.

(a) Give an example of a ceramic that shows superconductivity properties.
(b) Explain the difference in the electrical conductivity properties of the two conductors in Figure 1.
(c) How do scientists create a very cold condition to investigate the superconductivity phenomena?
Answer:
(a) Yttrium barium copper oxide ceramic, YBCO
(b) When temperature drops, the electrical resistance of metals that are not superconductors also drops but the resistance does not disappear even though at the lowest temperature 0 K When temperature drops, the electrical resistance of superconductor X also drops and the resistance disappears at a very low critical temperature (4 K) – close to absolute zero, 0 K.
(c) Cool the substance in liquid nitrogen or helium
