Laboratory Activity 4A (Synthesis of Nylon):
Aim: To synthesise nylon and study the properties of nylon produced.
Materials: 1,6-diaminohexane, C6H16N2, decanedioyl dichloride, C10H16Cl2O2, cyclohexane, C6H14, sodium hydroxide powder, NaOH and distilled water.
Apparatus: Glass rod, beaker, tweezer and measuring cylinder.
Procedure:
1. Dissolve 2 cm3 of decanedioyl dichloride, C10H16Cl2O2 in 50 cm3 hexane, C6H14 and label it as Solution A.
2. Dissolve 3 cm3 of 1,6-diaminohexane, C6H16N2 and 1 g of sodium hydroxide powder, NaOH in 50 cm3 of distilled water; label it as Solution B.
3. Using a glass rod, pour Solution B slowly into Solution A to avoid mixing.
4. Pull the layer formed between the two solutions using a tweezer and coil it around the glass rod as shown in Figure 4.5.
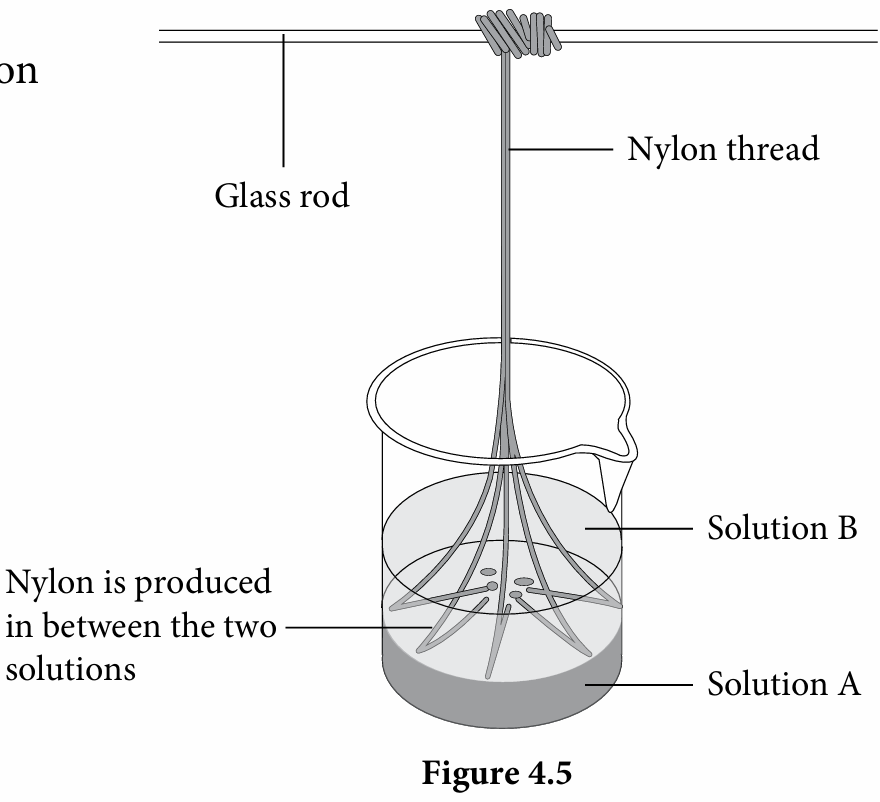
5. Wind the glass rod slowly to pull out the nylon until no more nylon is produced.
6. Wash the nylon produced on the glass rod with distilled water and dry.
Discussion:
1. Using the nylon thread produced, discuss:
(a) the strength of the nylon
(b) the elasticity of the nylon
(c) the resistance towards heat
2. State the uses of nylon.
Answer:
1. (a)
Nylon is exceptionally strong.
The fibres have excellent toughness.
Resistant to abrasion.
(b) Very elastic.
(c) Temperature resistant
2.
Uses of nylon:
• making clothes,
• reinforcement in rubber materials such as car tyres,
• as rope or thread,
• as a substitute for low-strength materials,
• plastic components in engine compartments of vehicles.
Aim: To synthesise nylon and study the properties of nylon produced.
Materials: 1,6-diaminohexane, C6H16N2, decanedioyl dichloride, C10H16Cl2O2, cyclohexane, C6H14, sodium hydroxide powder, NaOH and distilled water.
Apparatus: Glass rod, beaker, tweezer and measuring cylinder.
Procedure:
1. Dissolve 2 cm3 of decanedioyl dichloride, C10H16Cl2O2 in 50 cm3 hexane, C6H14 and label it as Solution A.
2. Dissolve 3 cm3 of 1,6-diaminohexane, C6H16N2 and 1 g of sodium hydroxide powder, NaOH in 50 cm3 of distilled water; label it as Solution B.
3. Using a glass rod, pour Solution B slowly into Solution A to avoid mixing.
4. Pull the layer formed between the two solutions using a tweezer and coil it around the glass rod as shown in Figure 4.5.

5. Wind the glass rod slowly to pull out the nylon until no more nylon is produced.
6. Wash the nylon produced on the glass rod with distilled water and dry.
Discussion:
1. Using the nylon thread produced, discuss:
(a) the strength of the nylon
(b) the elasticity of the nylon
(c) the resistance towards heat
2. State the uses of nylon.
Answer:
1. (a)
Nylon is exceptionally strong.
The fibres have excellent toughness.
Resistant to abrasion.
(b) Very elastic.
(c) Temperature resistant
2.
Uses of nylon:
• making clothes,
• reinforcement in rubber materials such as car tyres,
• as rope or thread,
• as a substitute for low-strength materials,
• plastic components in engine compartments of vehicles.
