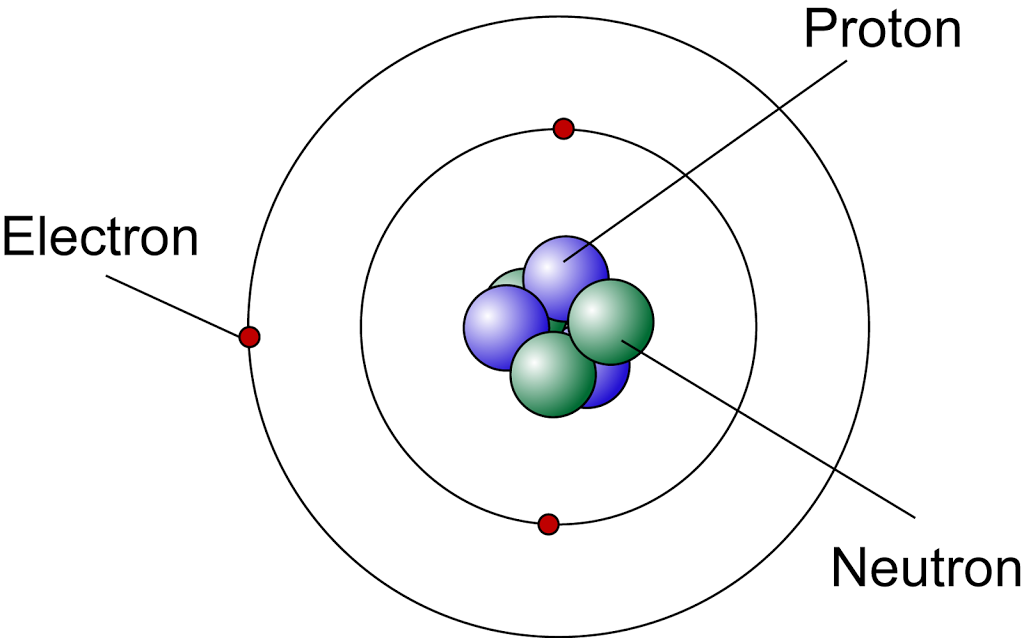
Modern Atomic Model
According to the modern atomic model,
- The central nucleus consists of protons and neutrons. It containing almost all the mass of the atom.
- the nucleus of an atom is very small compared to the size of the atom
- the electrons are orbiting outside the nucleus in the electron shells
- the electrons are moving in electron shells at a very high speed and we cannot determine the position of the electrons at a particular time
The Subatomic Particles of an Atom
- Atoms are made up of tiny particles called subatomic particles.
- An atom contains three types of subatomic particles:
- proton,
- neutron and
- electron,
- The proton and neutron form the nucleus at the centre of an atom. They are also called the nucleon of an atom.
- The electron moves around the nucleus at a very high speed.
- The nucleus is positively charged because of the presence of protons, which are positively charged. The neutrons are neutral.
- The symbols, charge and relative masses of proton, neutron and electron are as below.
| Particle | Symbol | Relative charge | Relative mass |
| Proton |
p |
+1 |
1 |
| Neutron |
n |
0 |
1 |
| Electron |
e |
-1 |
1/1840 |