Chemical Formlula of Ionic Compounds
2 Requirements to form the formula of an ionic compound
- Have at least 2 types of ions that contain opposite charge.
- The amount of positive charge/charges must be equal to the amount of negative charge/charges in the compound.
Example 1 – If the Charge of the Positive Ions = Charge of Negative Ions
Write the formula of each of the following compound
- Potassium bromide
- Sodium chloride
Example 2 – If the Charge of the Positive Ions ≠ Charge of Negative Ions
Write the formula of each of the following compound
- Calcium iodide
- Sodium oxide
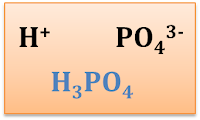
Example 3 – If there is more than 1 element in the ion
Write the formula of each of the compound
- Ammonium sulphate
- Zinc nitrate