Quiz Summary
0 of 10 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 10 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Answered
- Review
-
Question 1 of 10
1. Question
1 point(s)CHEM10203010
Which of the following is not a correct use of radioisotope?
CorrectIncorrect -
Question 2 of 10
2. Question
1 point(s)The maximum number of electrons can be filled into the second shell of an atom is _____.
CorrectIncorrect -
Question 3 of 10
3. Question
1 point(s)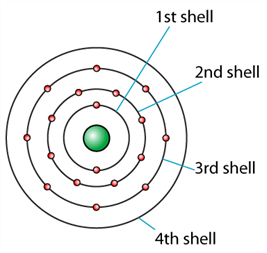
The diagram shows an atom with 18 electrons. If 1 more electron is added into the atom, the atom will fall into which shell?
CorrectIncorrect -
Question 4 of 10
4. Question
1 point(s)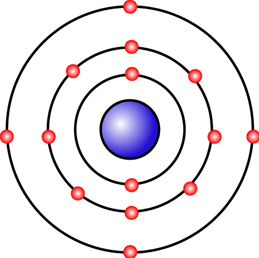
The figure shows an atom with 14 electrons. The electron arrangement of the atom is
CorrectIncorrect -
Question 5 of 10
5. Question
1 point(s)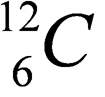
The figure shows the symbol of a carbon atom. Find the arrangement of the electrons of the carbon atom.
CorrectIncorrect -
Question 6 of 10
6. Question
1 point(s)One oxygen atom has 8 protons, 8 neutrons and 8 electrons. Which of the following statement is correct about oxygen atom?
CorrectIncorrect -
Question 7 of 10
7. Question
1 point(s)The table below describes the structures of four particles.
Particle O O2- Na Na+ Number of
protons8 8 11 11 Number of
neutrons8 8 Y 12 Number of electrons 8 X 11 Z What are the correct values of X, Y and Z?
CorrectIncorrect -
Question 8 of 10
8. Question
1 point(s)The table below shows the number of protons for elements P, Q, R, S and T.
Element Number of protons P 3 Q 8 R 12 S 11 T 20 Which of the following pair of elements have the same number of valence electrons in their atom?
CorrectIncorrect -
Question 9 of 10
9. Question
1 point(s)Table below shows the number of neutrons, protons and electrons of particles X and Y.
Particle
ZarahNumber of
neutrons
Bilangan neutronNumber of
proton
Bilangan protonNumber of
electrons
Bilangan elektronX 12 12 12 Y 12 12 10 Which of the following is true about X and Y?
CorrectIncorrect -
Question 10 of 10
10. Question
1 point(s)The nucleus of an atom contains
CorrectIncorrect
