Quiz Summary
0 of 11 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 11 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- Answered
- Review
-
Question 1 of 11
1. Question
1 point(s)Which of the following can be observed when an ammonia solution is added into a solution that contains copper(II) ions.
CorrectIncorrect -
Question 2 of 11
2. Question
1 point(s)A white precipitate formed when some aqueous ammonia is added to zinc chloride solution. Which of the following can be observed if the aqueous ammonia is added in excess?
CorrectIncorrect -
Question 3 of 11
3. Question
1 point(s)When gas X is directed through lead(II) nitrate solution, white precipitate is formed. Gas X may be
CorrectIncorrect -
Question 4 of 11
4. Question
1 point(s)Which of the following will give a colourless solution as the only product when reacting with hydrochloric acid?
CorrectIncorrect -
Question 5 of 11
5. Question
1 point(s)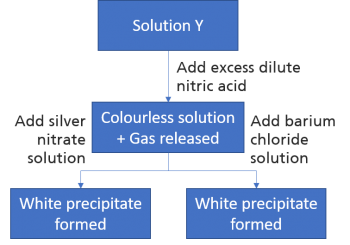
Solution Y contain more than 1 anion. The figure above shows a test done on solution Y. The anion in solution Y is
CorrectIncorrect -
Question 6 of 11
6. Question
1 point(s)Dilute nitric acid is added into a brownish solution Z. There are no changes occur. Silver nitrate solution is then added. A white precipitate formed. Solution Z might be
CorrectIncorrect -
Question 7 of 11
7. Question
1 point(s)Which of the following reagent can be used to test the presence of chloride ions in hydrochloric acid?
CorrectIncorrect -
Question 8 of 11
8. Question
1 point(s)Salt Y released a colourless gas when heated strongly. The residue produced is yellow when hot and white when cold. Which of the following is likely to be Y?
CorrectIncorrect -
Question 9 of 11
9. Question
1 point(s)Salt X is light green in colour. When heated strongly, a brownish gas which has pungent smell is released. The residue from the heating is also brown in colour. Salt S is probably
CorrectIncorrect -
Question 10 of 11
10. Question
1 point(s)Which of the following salts will produce an acidic gas when heated?
CorrectIncorrect -
Question 11 of 11
11. Question
1 point(s)Which of the nitrate salts below will form a metal oxide when heated strongly?
CorrectIncorrect
