Quiz Summary
0 of 7 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 7 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- Answered
- Review
-
Question 1 of 7
1. Question
1 point(s)Which one of the following oxides dissolves in water to form an acidic solution?
CorrectIncorrect -
Question 2 of 7
2. Question
1 point(s)Which of the following substance reacts with dilute nitric acid to produce hydrogen gas?
CorrectIncorrect -
Question 3 of 7
3. Question
1 point(s)Which of the following substance reacts with dilute nitric acid to produce carbon dioxide gas?
CorrectIncorrect -
Question 4 of 7
4. Question
1 point(s)The figure shows the apparatus set up for an electrolysis experiment. The ammeter will show a reading when
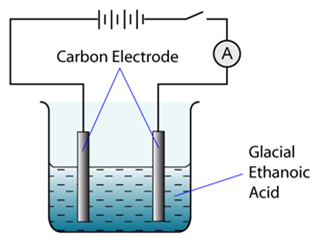 CorrectIncorrect
CorrectIncorrect -
Question 5 of 7
5. Question
1 point(s)Which of the followings are NOT true about hydrochloric acid?
CorrectIncorrect -
Question 6 of 7
6. Question
1 point(s)Which of the following statements are true about the reactions of dilute hydrochloric acid, sulphuric acid and nitric acid?
CorrectIncorrect -
Question 7 of 7
7. Question
1 point(s)Which of the following statements are true for all acids?
CorrectIncorrect
