Quiz Summary
0 of 9 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 9 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- Answered
- Review
-
Question 1 of 9
1. Question
1 point(s)Combustion of propane in excess oxygen can be represented by the equation
below.C₃H₈ + 5O₂ → 3CO₂ + 4H₂O ∆H = -y kJ/mol
Which of the following statements are true?
CorrectIncorrect -
Question 2 of 9
2. Question
1 point(s)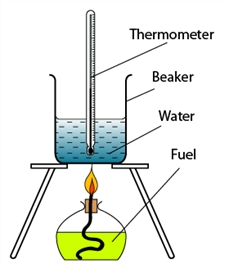
The figure shows the set-up of apparatus to determine heat of combustion of ethanol. A student found that the heat of combustion obtained is less than the theoretical value. What can be done to improve the experimental value?
CorrectIncorrect -
Question 3 of 9
3. Question
1 point(s)The burning of 0.6 g of X causes the temperature of 100 cm3 water to increase by 12oC. What is the heat of combustion of X?
[ relative molecular mass of X = 60; specific heat capacity of water = 4.2 J g-1 oC-1]CorrectIncorrect -
Question 4 of 9
4. Question
1 point(s)A student carries out an experiment to determine the heat of combustion of propanol. Which of the following information does he need in order to calculate the heat of combustion?
CorrectIncorrect -
Question 5 of 9
5. Question
1 point(s)Below are two thermochemical equations for the neutralization process.
KOH + HNO₃ → KNO₃ + H₂O ∆H = -57kJ/mol
KOH + CH₃COOH → CH₃COOK + H₂O ∆H = -55kJ/mol
The value of the heat of neutralization for both processes are different because
CorrectIncorrect -
Question 6 of 9
6. Question
1 point(s)In a neutralization reaction, 50 cm³ of 1.0 mole dm⁻³ nitric acid reacted with
50 cm³ of 1.0 mole dm⁻³ sodium hydroxide solution. Which of the following
acids can replace 50 cm³ of 1.0 mole dm⁻³ nitric acid to release the same quantity
of heat?CorrectIncorrect -
Question 7 of 9
7. Question
1 point(s)Which of the following acid releases the highest amount of heat when reacted with excess sodium hydroxide?
CorrectIncorrect -
Question 8 of 9
8. Question
1 point(s)When 100 cm³ of 1.0 mol dm⁻³ of sodium hydroxide is added to 100 cm³ of 1.0 mol dm⁻³ of hydrochloric acid, the temperature rises by 7°C. Which of the following mixtures will produce an increment of 14°C in temperature?
CorrectIncorrect -
Question 9 of 9
9. Question
1 point(s)When 50 cm³ of 1.0 mol dm⁻³ nitric acid is mixed with 50 cm³ of 1.0 mol dm⁻³ sodium hydroxide solution the temperature increases by 6.0 °C . What is the temperature change if the experiment is repeated using 50 cm³ of 2.0 mol dm⁻³ nitric acid with 50 cm³ of 2.0 mol dm⁻³ sodium hydroxide?
CorrectIncorrect
