Quiz Summary
0 of 12 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 12 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- Answered
- Review
-
Question 1 of 12
1. Question
1 point(s)Which of the following is true about the rate of a reaction?
CorrectIncorrect -
Question 2 of 12
2. Question
1 point(s)Which of the followings is not the unit of the rate of a reaction?
CorrectIncorrect -
Question 3 of 12
3. Question
1 point(s)The following chemical equation shows the reaction of calcium carbonate with sulphuric acid.
CaCO3(s) + H2SO4(aq) → CaSO4(aq) + CO2(g) + H2O(l)
Which of the followings are suitable to be used to find the average rate of the reaction.
CorrectIncorrect -
Question 4 of 12
4. Question
1 point(s)Which of the following methods can be used to find the rate of a reaction?
CorrectIncorrect -
Question 5 of 12
5. Question
1 point(s)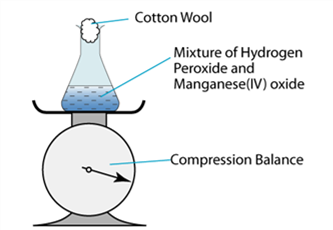
The diagram above shows the set-up of the apparatus used to investigate the decomposition rate of hydrogen peroxide. Which of the following is the graph obtained when the reading of the compression balance is plotted against time?
CorrectIncorrect -
Question 6 of 12
6. Question
1 point(s)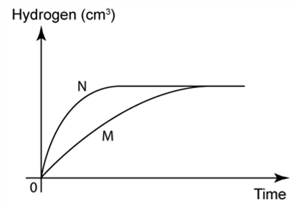
The figure shows the graph of the volume of hydrogen gas released against time for the reaction between granulated zinc and hydrochloric acid. The amount of zinc used is in excess. Graph M is obtained when 25 cm³ hydrochloric acid of concentration 1 mol/dm³. is used. Which of the following must be done to produce curve N?
CorrectIncorrect -
Question 7 of 12
7. Question
1 point(s)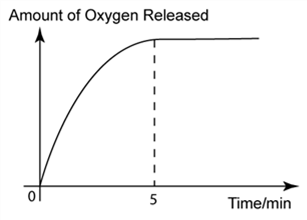
The graph below shows the volume of oxygen gas released over time in a chemical reaction. Why the volume of the oxygen remains unchanged after 5 minutes?
CorrectIncorrect -
Question 8 of 12
8. Question
1 point(s)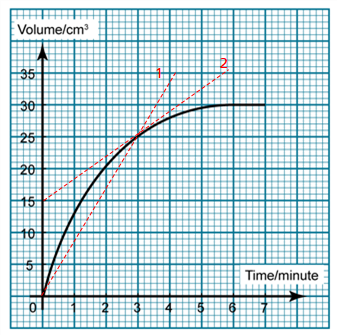
The graph shows the volume of carbon dioxide produced against time in a chemical reaction. Find the rate of reaction at 3 minutes.
CorrectIncorrect -
Question 9 of 12
9. Question
1 point(s)CHEM1003010
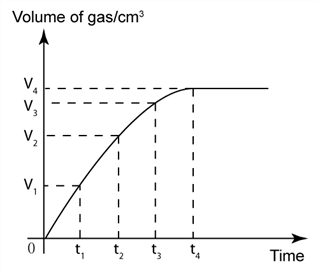
The graph shows the volume of gas released in a reaction against time. Which of the following statement is not true about the reaction?
CorrectIncorrect -
Question 10 of 12
10. Question
1 point(s)13.0g of zinc reacts completely with excess hydrochloric acid in 5 minutes. Find the average of the reaction. [ Relative atomic mass of Zinc = 65]
CorrectIncorrect -
Question 11 of 12
11. Question
1 point(s)2HCl(aq) + Na2S2O3(aq) → 2NaCl(aq) + S(s) + SO2(g) + H2O(l)
The equation above represents the reaction between dilute hydrochloric acid and sodium thiosulphate. Given that sulphur precipitate was produced after 40 seconds. What is the rate of the reaction?CorrectIncorrect -
Question 12 of 12
12. Question
1 point(s)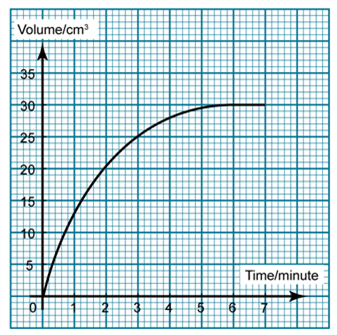
The graph shows the volume of carbon dioxide produced against time in a chemical reaction. Find the rate of reaction for the first 4 minutes.
CorrectIncorrect
