Quiz Summary
0 of 10 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 10 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Answered
- Review
-
Question 1 of 10
1. Question
1 point(s)The mass of an atom of element A is twice of the mass of an atom of carbon. Therefore its relative atomic mass is __________. [Relative Atomic Mass of Carbon = 12]
CorrectIncorrect -
Question 2 of 10
2. Question
1 point(s)What is the relative molecular mass of Calcium Hydroxide (Ca(OH)₂)?
( Ar: O = 16, H = 1; Ca = 40 )CorrectIncorrect -
Question 3 of 10
3. Question
1 point(s)Given that the formula of a compound is Na₂XO₄ and its relative molecular mass is 142. Find the relative atomic mass of element X?
(Relative Atomic Mass: O = 16; Na = 23 )CorrectIncorrect -
Question 4 of 10
4. Question
1 point(s)The nucleon number of bromine is 80. What is the relative atomic mass of bromine?
CorrectIncorrect -
Question 5 of 10
5. Question
1 point(s)The number of electrons of ion X2+ is 18. The number of neutrons of atom X is 20. The relative atomic mass of X is probably
CorrectIncorrect -
Question 6 of 10
6. Question
1 point(s)One mole of substance is defined as the quantity of a substance that contains the same number of particles in 12.000g of _____________.
CorrectIncorrect -
Question 7 of 10
7. Question
1 point(s)Which contains more atoms, 1 mol of helium or 1 mol of uranium?
[ RAM: He=4; U=238 ]CorrectIncorrect -
Question 8 of 10
8. Question
1 point(s)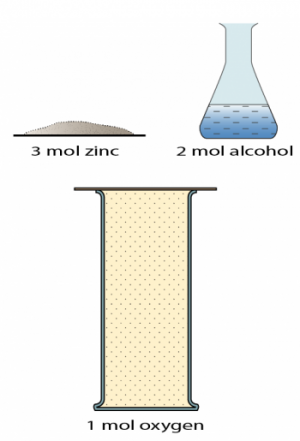
Which of the followings are true about the substance in the diagram?
CorrectIncorrect -
Question 9 of 10
9. Question
1 point(s)Find the number of atoms in 1.4 mol of copper metal.
[Avogadro constant = 6.02 x 10²³]CorrectIncorrect -
Question 10 of 10
10. Question
1 point(s)How many moles of magnesium that contain 2.408 x 10²³ of magnesium atom? [Avogadro number = 6.02 x 10²³]
CorrectIncorrect
