Quiz Summary
0 of 11 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 11 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- Answered
- Review
-
Question 1 of 11
1. Question
1 point(s)CHEM10202020
Substance X exists as a liquid at a temperature of 85°C. What is the most likely melting point and boiling point of substance X?CorrectIncorrect -
Question 2 of 11
2. Question
1 point(s)CHEM10202020
The table below shows the meting point and boiling point of substances P, Q, R and S. Which of the substance is solid at room temperature (25 °C)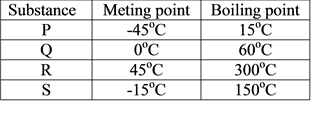 CorrectIncorrect
CorrectIncorrect -
Question 3 of 11
3. Question
1 point(s)CHEM10202030
Which of the following statement is true about Neils Bohr’s atomic model?CorrectIncorrect -
Question 4 of 11
4. Question
1 point(s)CHEM10202040
Which of the following match is not true?CorrectIncorrect -
Question 5 of 11
5. Question
1 point(s)CHEM10202050
Which of the following shows the correct relative mass and charge of the subatomic particles?CorrectIncorrect -
Question 6 of 11
6. Question
1 point(s)CHEM10202060
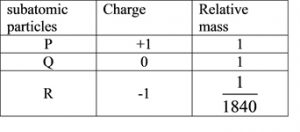
The table above shows the properties of subatomic particles P, Q and R. Name P, Q and R.
CorrectIncorrect -
Question 7 of 11
7. Question
1 point(s)CHEM10202070

The figure above is the symbol of sulphur element. Which of the following is true about the symbol?
CorrectIncorrect -
Question 8 of 11
8. Question
1 point(s)CHEM10202080
In an atom, the number of electrons is equal to theCorrectIncorrect -
Question 9 of 11
9. Question
1 point(s)CHEM10202090
The number of subatomic particles in particle P and Q is as below.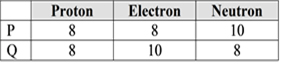
Which of the following statement is true about particle P and Q?
CorrectIncorrect -
Question 10 of 11
10. Question
1 point(s)CHEM10202100
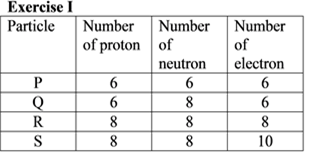
Based on the information in the table above, which of the following pair of particles are isotopes?
CorrectIncorrect -
Question 11 of 11
11. Question
1 point(s)CHEM10202110
The table below shows the melting point and boiling points of five compounds P, Q, R, S and T. Which substance exists as a liquid at room temperature?Substance Melting point(°C) Boiling point(°C) P -185 -18 Q -40 45 R 10 500 S 45 900 T 800 3000 CorrectIncorrect
