Quiz Summary
0 of 10 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 10 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Answered
- Review
-
Question 1 of 10
1. Question
1 point(s)Which of the following diagram shows the correct apparatus set up to electroplate an iron with copper?
CorrectIncorrect -
Question 2 of 10
2. Question
1 point(s)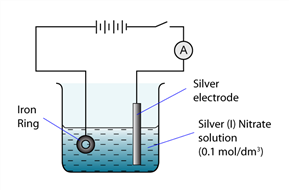
The figure shows the apparatus set up prepared by a student to electroplate an iron ring with silver. A current of 0.3A flows through the wire. However, the student found that this experiment fails at the end of the experiment. This is because
CorrectIncorrect -
Question 3 of 10
3. Question
1 point(s)You are required to electroplate a metal spoon with chromium. Which of the following substances are suitable to be used as anode and electrolyte in the process?
CorrectIncorrect -
Question 4 of 10
4. Question
1 point(s)Which of the followings are true about the metals and the method to extract (Method) these metals from their ore in industry?
CorrectIncorrect -
Question 5 of 10
5. Question
1 point(s)The figure shows the setup of apparatus to purify copper. Which of the following is true about this process?
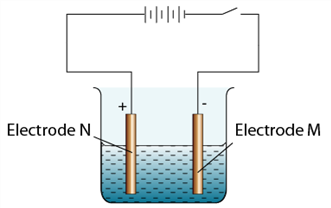 CorrectIncorrect
CorrectIncorrect -
Question 6 of 10
6. Question
1 point(s)Which of the following is true about the product produced at anode and cathode and the change of concentration of the acid when dilute sulphuric acid is electrolysed by using platinum electrodes?
CorrectIncorrect -
Question 7 of 10
7. Question
1 point(s)The figure shows the apparatus set up for the electrolysis of solution M. Gas X and Y is produced at anode and cathode respectively. The volume of gas Y is double the volume of gas X. Solution M might be
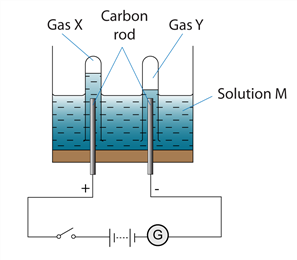 CorrectIncorrect
CorrectIncorrect -
Question 8 of 10
8. Question
1 point(s)Which of the solutions produces oxygen gas at anode when it is electrolysed with carbon electrode?
CorrectIncorrect -
Question 9 of 10
9. Question
1 point(s)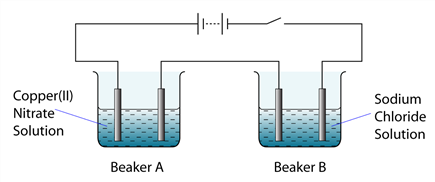
The diagram above shows the apparatus set up for the electrolysis of copper (II) nitrate solution in beaker A and dilute sodium chloride in beaker B using carbon electrodes. Which of the following is the product formed at the cathode in beaker A and beaker B?
CorrectIncorrect -
Question 10 of 10
10. Question
1 point(s)Dilute copper (II) sulphate solution is electrolysed by using copper electrodes. What is the product formed at anode and cathode?
CorrectIncorrect
