Quiz Summary
0 of 10 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 10 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Answered
- Review
-
Question 1 of 10
1. Question
1 point(s)The reaction between magnesium and iron(II) sulphate is represented by the following equation.
Mg + FeSO₄ → MgSO₄ + Fe ∆H = -189 kJ mol⁻¹
Which of the statements below are TRUE about the reaction?
CorrectIncorrect -
Question 2 of 10
2. Question
1 point(s)The energy change during the precipitation of calcium sulphate is shown in the equation below:
Ca²⁺ + SO₄²⁻ → CaSO₄ ∆H = -1435.5 kJ/mol
What is the energy change when 400cm³ of calcium chloride, CaCl₂, 0.5 mol/dm³ reacts with 200cm³ of ammonium sulphate , (NH₄)₂SO₄, 0.5 mol/dm³.
CorrectIncorrect -
Question 3 of 10
3. Question
1 point(s)The following thermochemical equation shows the formation of lead (II) iodide.
Pb²⁺ + 2I- → PbI₂ ∆H = -y kJ/mol
The -y kJ/mol is called the heat of
CorrectIncorrect -
Question 4 of 10
4. Question
1 point(s)The following thermochemical equation represents the formation of ammonia gas.
2N₂ + 3H₂ → 2NH₃ ∆H = -xkJ/mol
Which of the following statements are true for the reaction?
CorrectIncorrect -
Question 5 of 10
5. Question
1 point(s)Below is a thermochemical equation.
2Na + 2H₂O → 2NaOH + H₂ ∆H = -x kJ/mol
What is the amount of heat released if 0.5 mol of sodium reacts with sufficient water?
CorrectIncorrect -
Question 6 of 10
6. Question
1 point(s)
The figure shows a burning match. By striking a match, a chemical reaction is initiated and ignites the match. Which of the following statements about the chemical reaction is correct?
CorrectIncorrect -
Question 7 of 10
7. Question
1 point(s)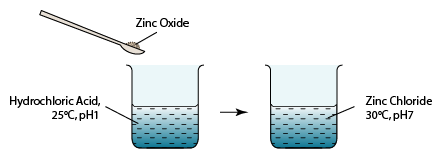
Diagram above shows an experiment in which zinc oxide powder is added to dilute hydrochloric acid. Which of the following is true about this reaction?
CorrectIncorrect -
Question 8 of 10
8. Question
1 point(s)HCl + NaOH → NaCl + H₂O
The equation above represents the reaction between hydrochloric acid and sodium hydroxide. Given that this is an exothermic reaction. Which of the following statements are true about this reaction?CorrectIncorrect -
Question 9 of 10
9. Question
1 point(s)Which of the following chemical reactions involving the release of heat to the surroundings?
CorrectIncorrect -
Question 10 of 10
10. Question
1 point(s)Which of the followings are endothermic reactions?
CorrectIncorrect
