Quiz Summary
0 of 10 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 10 questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Answered
- Review
-
Question 1 of 10
1. Question
1 point(s)Which of the following is correct about the colour of halogen when dissolve in water?
CorrectIncorrect -
Question 2 of 10
2. Question
1 point(s)2 reactants A and B are mixed with tetrachloromethane. Which of the following is correct about the reactants and the colour of tetrachloromethane at the end of the experiment?
Reactants A and B Colour of Tetrachloro-methane A Potassium iodide solution + chlorine water Colourless B Potassium chloride solution + bromine water Brown C Potassium iodide solution + bromine water Purple D Potassium bromide solution + iodine water Brown CorrectIncorrect -
Question 3 of 10
3. Question
1 point(s)Metal which placed higher in the electrochemical series
CorrectIncorrect -
Question 4 of 10
4. Question
1 point(s)When an ion of metal is displaced by a more reactive metal, the ion of metal
CorrectIncorrect -
Question 5 of 10
5. Question
1 point(s)The following ionic equation shows the reaction between iron(II) sulphate solution and acidified potassium dichromate(VI) solution
Cr₂O₇²⁻ + 14H+ + 6Fe²⁺ → 2Cr³⁺ + 6Fe³⁺ + 7H₂O
Which of the following statements are true about this reaction?
CorrectIncorrect -
Question 6 of 10
6. Question
1 point(s)The figure shows an experiment carried out for iron(II) sulphate. Which of the following may be solution X?
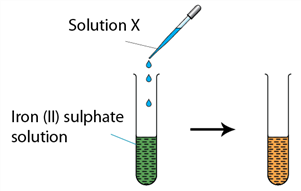 CorrectIncorrect
CorrectIncorrect -
Question 7 of 10
7. Question
1 point(s)The figure shows an experiment to study the redox reaction on iron (III) sulphate solution. The orange colour of iron (III) sulphate solution turn green when a few drops of solution X is added into it. Possibly, solution X is
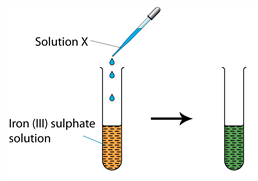 CorrectIncorrect
CorrectIncorrect -
Question 8 of 10
8. Question
1 point(s)2Fe²⁺ + Cl₂ → 2Fe³⁺ + 2Cl⁻
The ionic equation above shows the redox reaction between iron(II) sulphate and chlorine. Which of the following is true about the reaction?CorrectIncorrect -
Question 9 of 10
9. Question
1 point(s)The following ionic equation represents a redox reaction
2Fe²⁺ + Br₂ → 2Fe³⁺ + 2Br⁻
Which of the following statements is true?
CorrectIncorrect -
Question 10 of 10
10. Question
1 point(s)Cr₂O₇²⁻ + 14H⁺ + 6Fe²⁺ → 2Cr³⁺ + 6Fe³⁺ + 7H₂O
The ionic equation above shows the reaction between iron(II) sulphate solution and acidified potassium dichromate(VI) solution. Which of the following statements are true about this reaction?
CorrectIncorrect
