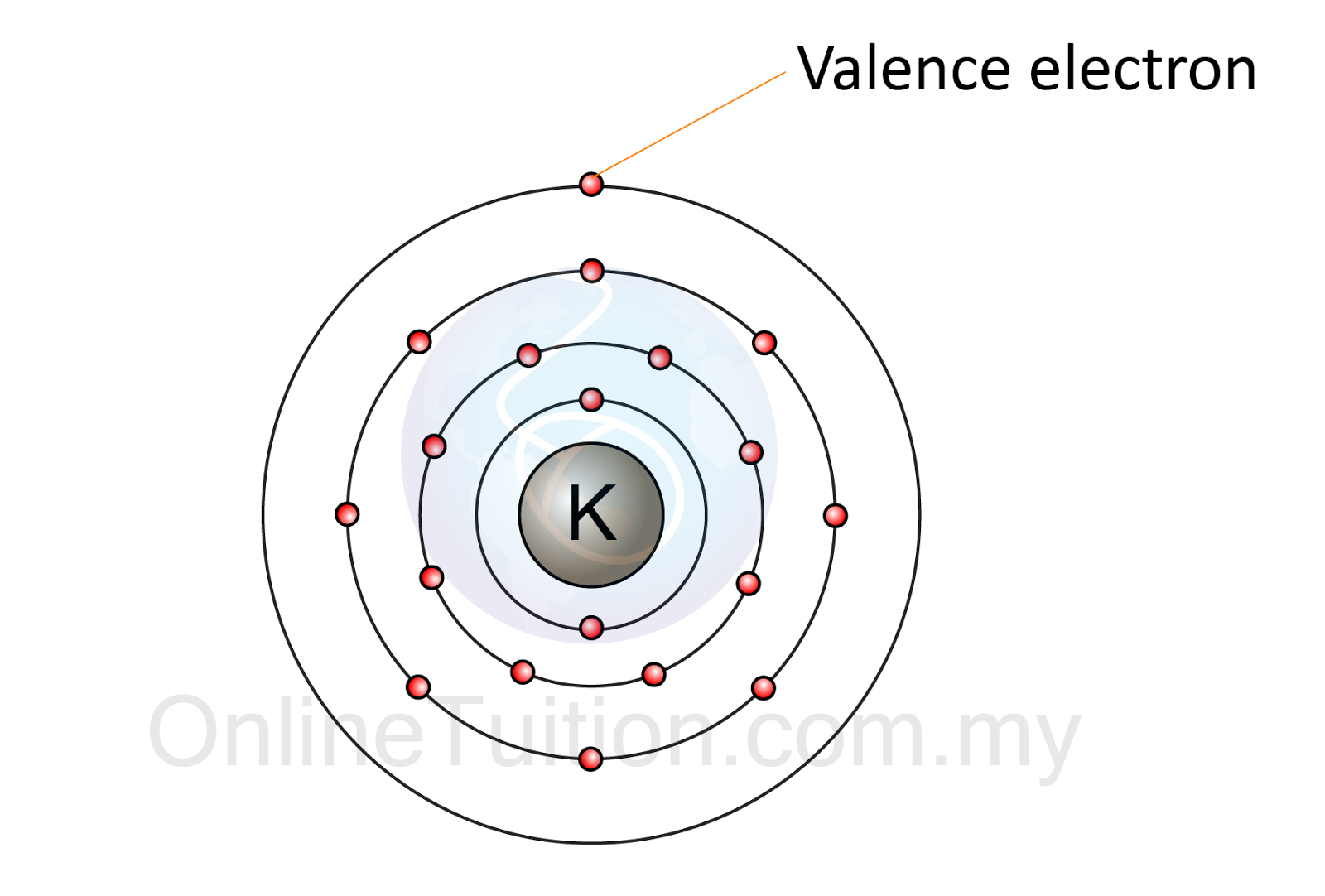
Valence Electron
Valence electrons are the electrons in the outermost shell.
- The electrons in the outermost shell of an atom are called valence electrons.
- The valence electrons have great significance in determining the chemical properties of an atom.
- Elements with the same number of valence electron have the same chemical properties.
Example:
Given that a sodium atom has 11 protons 12 neutrons. Find the number of valence electron in a sodium atom.
Answer:
For an atom,
Number of electrons = number of protons = 11
Electron arrangement of sodium = 2.8.1
Therefore, sodium has 1 valence electron.